Stem Cell Therapy used to Treat Spina Bifida in Babies
Beverly A. Davis and Vincent S. Gallicchio*
Department of Biological Sciences, College of Science, Clemson University, Clemson, South Carolina, USA, 29636
*Corresponding author: Vincent S. Gallicchio, Department of Biological Sciences, College of Science, Clemson University, Clemson, South Carolina, USA, 29636
Citation: Davis BA and Gallicchio VS. (2024) Stem Cell Therapy Used to Treat Spina Bifida in Babies. J Stem Cell Res. 5(1):1-7.
Received: April 3, 2024 | Published: April 18, 2024
Copyright© 2024 genesis pub by Dav8is BA, et al. CC BY-NC-ND 4.0 DEED. This is an open-access article distributedunder the terms of the Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International License.,This allows others distribute, remix, tweak, and build upon the work, even commercially, as long as they credit the authors for the original creation.
Abstract
Spina Bifida is a rare congenital anomaly that affects children across the world daily. This birth defect is caused by the improper fusing of the spinal cord while in the womb and often leads to spinal cord and nerve damage. Children with Spina Bifida often have a shorter life expectancy and have many medical issues, including paralysis and urinary complications. Traditional treatment is for therapeutic procedures only after birth, and there is currently no cure for the disease. More recently, stem cells have been used to treat the condition in hopes of repairing and healing the spinal cord stem cells because the regenerative properties present in stem cells allow for constant self-renewal of the cells. This literature review aims to analyze the current methods used for treating Spina Bifida, including clinical trials and animal trials. The use of stem cells has been proven to show promising signs in treating children with Spina Bifida. Stem cell therapy is costly and can present several ethical issues, including banning federal-funded stem cell research. However, stem cell use is projected to become more prevalent in medicine as technology advances. It is evident that stem cells will likely lead researchers and physicians toward a cure for Spina Bifida in the future.
Keywords
Spina Bifida; Stem Cells; Mesenchymal; Stem Cell Patch
Abbreviations
FDA: Food and Drug Administration; MMC: Myelomeningocele; NTD: Neural tube defects; HKAFO: Hip-Knee-Ankle-foot Orthoses; CIC: Clean Intermittent Catheterization; UTI: Urinary Tract Infection; PT: Physical Therapy; OT: Occupational Therapy; UC Davis: University of California, Davis; Cure clinical trial: Cellular therapy for In Utero Repair of Myelomeningocele; CIRM: California Institute for Regenerative Medicine; CDC: Center for Disease Control; MSC: Mesenchymal Stem Cells; P-MSC: Placental mesenchymal stem cells; GMP: Good Manufacturing Practice.
Introduction
Spina Bifida is something that something has been affecting children since the start of humanity. Spina Bifida is a birth defect in which the spine and spinal cord of the child do not properly develop and fuse while in the womb, more specifically, the neural tube of the spine. This condition can also be referred to as a split spine [1]. This defect results from many factors, the primary ones being genetic, nutritional, and environmental factors while the baby is in the womb.
There are different types of Spina Bifida. The mildest form is Spina Bifida occulta. In this form, the patient's skin covers the defeat, and there are no skin protrusions. The second form is Meningocele; this form affects the meninges, which cover the spinal and protect the brain. A sac, also referred to as the meningocele, is pushed by the meninge, hence, mild nerve damage occurs and results in mild disability. The final type of Spina Bifida is Myelomeningocele; this is the most severe form of defeat and often what people think of when they refer to Spina Bifida. In this form, the meninges and spinal cord protrude through the baby's back. This results in severe nerve damage, hence significant disability, including paralysis, neurological complications, and urinary issues [2].
The effects of improper spinal cord development are that the spinal cord tissue protrudes the skin, creating a hump-like structure or abnormal birthmark along the spinal cord on the back of the child. These defects can result in spinal cord and nerve injury, which can result in parallelization and neurological defects [1].
Figure 1: A visual depiction from the CDC of the distinct differences between the three types of Spina Bifida [3].
Epidemiology
Spina Bifida is very rare, with less than 200,000 cases per year. Although Spina Bifida is rare, it is still prevalent in the number of individuals affected each year. According to the CDC, Hispanic women have the highest rate of having a child affected by Spina Bifida. 3.80 per 10,00 births compare Hispanic births to non-Hispanic births with 3.09 per 10,000 births. African Americans are at least risk, with 2.73 children affected per 10,000 births. 1 in every 2,578 births is a child that is born with Spina Bifida [1]. The mother's health also plays an essential role in whether the child will develop this defeat; a study shows that women who are diabetic and obese are significantly more at risk of having a child with Spina Bifida [4].
Pathology
Spina Bifida is a congenital anomaly because it is identified before or at birth. This condition occurs when the neural tube of the spinal cord has an improper and incomplete development. More specifically, it is caused by the underdevelopment of ectodermal, mesodermal, and neuroectodermal tissues in the body [5].
Etiology
Spina Bifida happens because of improper closure of the posterior spinal area; this occurs between day 17 and day 30 of fetal development. The neuralization of this occurs in two phases: primary and secondary. In primary neutralization, the neural tube forms the spinal cord and the brain. In secondary neutralization, the caudal structures of the neural tube are formed. The development of the caudal is essential because on day 26 of development, the degree of Spina Bifida present depends on the severity of the spinal dysraphism and if there is an NTD present [6]. The neural tubes are essential because they are responsible for developing the baby's spinal cord and brain and neurological functions.
Environmental and genetic factors can also affect this condition. Some factors that can increase the chances of a baby developing this condition include the mother being diabetic, overweight, or having a history of Spina Bifida. Some other factors include the mother's low folic acid intake during pregnancy. A mother adding folic acid to her diet when pregnant can reduce the chances of NTDs. It is essential that during pregnancy, the mother regularly schedules appointments with her doctor because early intervention is possible for this defect [7].
Current Treatment & Care
Spina Bifida treatment is minimal due to the chronic nature of the condition. Spina Bifida can be detected when the baby is still in the womb via ultrasound, allowing for parents to understand treatment options before birth of the child. If not identified in the womb but within 48 hours post-birth, specifically as MMC. In that case, surgery can be performed to put the spinal cord as well as exposed or damaged tissue in the place it originally should have been. This procedure will stop the Spina Bifida from becoming more severe but will still leave the patient with many complications. Performing this surgery after 72 hours post-birth is extremely dangerous and will be rarely performed by physicians [8].
Moreover, this surgery reduces the chances of the child developing hydrocephalus in the future, as well as the spinal cord from facing more trauma in the future. Although there are benefits to this surgery, including the possibility for gained strength and muscle mobility, there are also risks associated. A significant risk from this surgery is the spinal cord getting tethered.
Physiotherapy is a widespread current practice used for Spina Bifida for pain management and increasing mobility and function. Physiotherapy aims to improve movement and, more importantly, help the child gain independence. This includes daily exercise and using HKAF, stabilizing the knee, foot, and ankle. Children might also need to utilize crutches for additional support and relieve the stress on the legs [9].
Treatment of bladder and bowel issues is essential to the current treatment of Spina Bifida. Due to nerve damage, many children with Spina Bifida face complications with controlling their bladders. As a result, many patients rely on CIC to effectively drain urine, which is essential for protecting the kidneys and preventing UTIs [10].
Children with Spina Bifida will need special accommodations at school. This includes wheelchair access as well as assistive technology available in the classroom. Children with Spina Bifida often need to be seen for care by a wide variety of care by various specialties because Spina Bifida affects many symptoms in the body. Specialty physicians who often work with these patients include physical medicine, neurology, neurosurgical, urology, orthopedics, PT, OT, and nutrition [8].
Why Stem Cells?
Stem cells can heal because of the regenerative properties they process, which allows for stem cell renewal. Healthy stem cells will be generated in the future, and the stem cells will keep producing in the body. Regarding Spina Bifida, stem cells can heal the opening of the spinal at the of the defeat. The treatment of stem cells for Spina Bifida seems more effective than any other traditional methods used for this condition. Moreover, although this type of stem cell therapy is highly effective, more therapies, such as water aerobics, yoga, acupuncture, and OT, have increased the patient's quality of life [9].
Animal Studies with Stem Cells and Spina Bifida
Several animal studies have been tested with myelomeningocele Spina Bifida with the use of stem cells. These animal studies were performed in the pre-clinical trials. The first model was a surgical model that used rabbits and chicks with MMC stem cells. 62% of the animals survived in the in-utero application. However, survival rates and stem cell application have no significant effect [10].
A rat model, a non-surgical model, was also performed. Retinoic acid was injected in pregnant mice, as well as an intra-amniotic injection of allogeneic amniotic fluid. Both surgical and non-surgical options were tested and were deemed to be feasible, safe, and efficient for in-utero stem cell transplantation for Spina Bifida. Animals were seen to have an increase in lower limb function. However, there is no data for large animals. However, at the time of this review, human trials have yet to be started with the model specifically [10].
Clinical Trials – UC Davis
UC Davis performed fetal surgery with stem cell therapy for a baby in the womb with Spina Bifida. Marking this surgery as the first in-utero stem cell treatment for Spina Bifida in the world is giving promise to parents whose children are diagnosed with Spina Bifida before birth. This trial was given FDA approval for the enrollment of human patients [11].
The CuRe trial was launched in 2021 in hopes of performing in-utero repair of the MMC on babies diagnosed with Spina Bifida while in the womb. This trial's goal is to reverse Spina Bifida, with the end goal of curing the condition soon. Lead scientist Dr. Diana Farmer leads the clinical trial. In this trial, the Spina Bifida will be treated before the baby is born and still in the womb. The surgery will enter the mother's womb and put a stem cell patch on the area where the Spina Bifida is present [12]. This is done in hopes that the stem cells will be able to heal the defeat before the child is born.
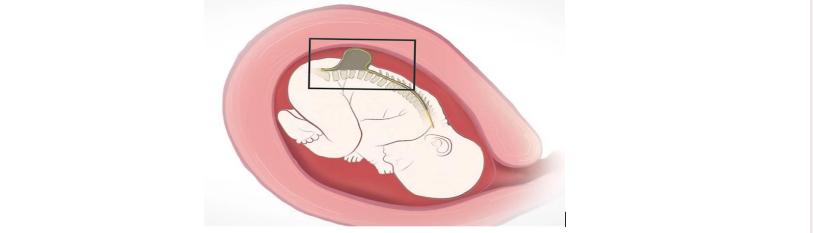
The process of developing the stem cell patch placed on the fetus's spinal cord is an extensive four-day process. The stem cell patch is made in a GMP laboratory to ensure the quality and creditability of the patch. The patch is made from MSC from the placental tissue. Once the surgery takes place at 26 weeks gestation. The mother has an incision made at her uterus, allowing for surgeons to see the baby and the spinal cord defeat [11]. The patch is carefully placed on the defeat with the use of a microscope due to the small size of the fetus [13]. Directly after the patch is placed, the mother’s incisions are closed, allowing for the tissue to begin repairing and regenerating.
At 36 weeks of gestation, in September 2021, the baby was born. The baby named Robbie could move lower extremities, a promising sign. The baby and mother were in healthy condition, and Robbie showed no signs of Spina Bifida. Robbie shows no signs of Spina Bifida after birth, the first time this has ever been done in the world. Robbie continues to be monitored regarding walking and potty training for 30 months [14].
Figure 2: Depiction of surgeon placing stem cell patch of the area of defeat on the fetus in the womb in the UC-Davis Clinical trial [11].
Future Directions
The future of treatment of Spina Bifida with stem cell therapy is promising, UC Davis was awarded 8.9 million dollars in grants from CIRM. Additionally, Shiner Children’s matched this grant with 5.9 million dollars. In total CIRM granted 15 million dollars to three different clinical-stage research programs focusing on regenerative medicine. The purpose of this grant is aiming to advance stem cell and gene therapy advanced. With the award of this grant, the CuRe trial will enter phase 2 of the clinical trial, which will aim to improve mobility in patients and improve bowel and bladder control. This will allow research to evaluate the successes of the clinical trial and look for improvements. CIRM is aiming to accelerate the use of stem cells in soon and extend the trial. There is an urgency to treat patients with unmet needs who need treatment. It is currently reported that there is currently 5.5 billion in funding in over 150 active stem cell programs [15].
However, regarding future direction, the United States has many regulations when it comes to use of stem cells in medical treatment. Federal regulation the use of stem cell sense hindering research and development for treatment. This is an obstacle that other countries do not have to face at such an intense measure allowing for options that include regenerative therapy. In 2001, under the Presidency of George W. Bush, the United States Federal Government put ban on new research that is federally funded going towards embryonic stem cell research [16]. Due to this federal ban placement, it significantly put the United States behind in the field of stem cell research. As a result, stem cells research needs to be privately funded, which is a challenging task and available to less of the population.
Conclusion & Summary
The use of stem cells to treat Spina Bifida is becoming an option for more children as clinical trials expand across the United States. Spina Bifida is a rare and chronic condition that effects children both cognitively and physically. This condition occurs when the spinal cord and neutral tube do not develop and fuse properly, therefore leaving the spinal cord and nerve endings exposed in some cases. This often leaves the child with many complications such as paralysis, neurological disorders, and urinary complications. Fortunately, Spina Bifida can be detected through ultrasound which allows for early intervention and treatment for the child as early as when still in the womb. Stem cell therapy is seen to be more effective to improve the quality of life of the patient more than the traditional treatment for the birth defect as it can be performed when the child is still in the womb.
The case study at UC Davis, a stem cell patch was placed on the spine of the baby who was still in womb. When the baby was born, it was normal birth and the baby present no complications associated with Spina Bifida. The mother also had no complications. This is the first time this has been done in world, making a large advancement in the medical system. It guides medical professional towards the cure of Spina Bifida, something that would change the lives of many children.
The future direction of stem cell therapy for Spina Bifida is promising as many private clinical trials are being funded in the hopes of finding a cure for this chronic defeat. The clinical trial team at UC Davis is planning to start treatment on new patients soon. However, the United States does have a ban on the federally funded use of stem cells putting the United States at a disadvantage.

