The Use of Stem Cells in the Treatment of Sensorineural Hearing Loss
Aubrey C Keller and Vincent S. Gallicchio*
Department of Biological Sciences, College of Science, Clemson University, Clemson, South Carolina, USA
*Corresponding author: Vincent S Gallicchio, Department of Biological Sciences, College of Science, Clemson University, Clemson, South Carolina, USA
Citation: Keller AC, Gallicchio VS. (2021) The Use of Stem Cells in the Treatment of Sensorineural Hearing Loss. J Stem Cell Res. 2(2):1-10.
Received: April 23, 2021 | Published: May 10, 2021
Copyright© 2021 genesis pub by Keller AC, et al. CC BY NC-ND 4.0 DEED. This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International License., This allows others distribute, remix, tweak, and build upon the work, even commercially, as long as they credit the authors for the original creation.
DOI: https://doi.org/10.52793/JSCR.2021.2(2)-S1
Abstract
Hearing loss affects millions of Americans in every age group, and it can be either conductive or sensorineural. Sensorineural hearing loss (SNHL) specifically has etiologies including trauma, infectious conditions, ototoxin exposure, and trauma. This type of hearing loss affects the conversion of mechanical sound to an electrical signal within the inner ear, and profound SNHL can usually only be treated with a cochlear implant. Neural stem cells (NSCs), embryonic stem cells (ESCs), mesenchymal stem cells (MSCs), and induced pluripotent stem cells (iPSCs) are all promising candidates for the treatment of SNHL. They have the potential to be induced to differentiate into sensory hair cells and spiral ganglion neurons (SGNs) in damaged cochleae. However, more research is still needed to improve the protocol for differentiating stem cells into hair cells and SGNs.
Keywords
Hearing loss; Stem cells; Inner ear; Hair cells; Spiral ganglion neurons
Introduction
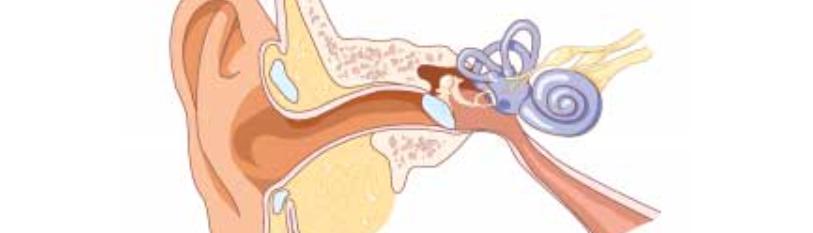
Hearing loss is one of the most common disabilities afflicting Americans. Approximately 30 million Americans or 12.7% of Americans, 12 years and older were estimated to have suffered from bilateral hearing loss from 2001 through 2008. This number increased to 48.1 million Americans when including individuals suffering from unilateral hearing loss. Furthermore, the prevalence of hearing loss increases with each age decade [1]. Hearing loss is classified as either conductive, sensorineural, or mixed type. Conductive hearing loss involves the tympanic membrane and the middle ear, and this type interferes with transmitting sound and converting it to mechanical vibrations. Meanwhile, sensorineural hearing loss (SNHL) affects the conversion of mechanical sound to electrical signals in the inner ear or the auditory nerve.
Sensorineural hearing loss can have a multitude of causes, including trauma, noise exposure, ototoxin exposure, age, and infectious conditions such as meningitis. As for the treatment of SNHL, for mild to moderate hearing loss, there is evidence that conventional hearing aids are effective in improving hearing [2]. However, cochlear implants are typically the only current treatment option for cases of profound bilateral SNHL that cannot be corrected with hearing aids and severe asymmetric SNHL [3]. Cochlear implants work by replacing the function of sensory hair cells that are no longer able to convert mechanical sound to electrical impulses. Thus, the implants bypass the damaged hair cells by directly transmitting the electrical signals to the auditory nerve. While these cochlear implants have been shown to restore some degree of auditory function, there is still low evidence regarding improvement in post-operative speech outcomes and improvement in hearing-related quality of life [4].
The primary reason for the permanence of hearing loss is that the mammalian cochlea lacks regenerative capacity. In mature mammalian cochleae, virtually no mechanosensitive hair cell regeneration is detectable. In contrast, the mammalian vestibular organs can regenerate hair cells in a limited capacity. The reason for the lack of regenerative capacity in the cochlea could be that the differentiated cell types that contribute to the structure of the organ of Corti lose their regenerative and proliferative ability during post-natal maturation [5]. This explanation has led to the hope that stem cells from other sources can repair damage to the cochlea that cannot repair itself. Stem cells have the marked ability of self-renewal as well as the capacity to differentiate into mature cells of a particular tissue [6]. The first type of stem cell is totipotent, which derive from the zygote and are able to form the embryo and trophoblasts of the placenta. Cells derived from the inner cell mass (ICM) of the blastocyst are considered to be pluripotent, and these are able to differentiate into nearly all cells arising from the three embryonic germ layers. Included in pluripotent cells in embryonic stem cells. Most of the body’s tissues have multipotent stem cells, which are capable of producing a limited range of differentiated cell lineages depending on their location. The final type of stem cells is unipotent, which are capable of generating only one specific cell type [7]. Research into these stem cells is clinically significant for SNHL because of the stem cells’ potential to replace lost or damaged mechanosensory hair cells or the spiral ganglion neurons [24].
This article aims to review the current literature about four different stem cell types with potential for application in stem cell therapy for sensorineural hearing loss, including key animal studies, in-vitro work, and a human clinical trial. The four stem cell therapies we will discuss in the context of repairing SNHL are as follows: neural stem cell therapy, embryonic stem cell therapy, mesenchymal stem cell therapy, and induced pluripotent stem cell therapy.
Neural Stem Cell (NSC) Therapy
Neural stem cells (NSCs) are multipotent stem cells that can differentiate into neurons or glial cells isolated from the hippocampus and cerebral cortex [8]. Several studies have shown promise in using these NSCs in treating sensorineural hearing loss by replacing spiral ganglion neurons or by replacing inner ear hair cells. For example, in a clinical study in Japan that investigated NSCs’ use for regenerating the inner ear, the research team estimated survival of NSCs in the cochlea. Though these NSCs successfully integrated into the inner ear’s sensory epithelia, there was an insufficient number of integrated cells and it was uncertain whether there was differentiation into the hair cell [9]. The same team did a similar experiment in 2004 to determine an optimal procedure for differentiating NSCs into hair cells. The team transplanted rat NSCs into explants of rat inner ear; the NSCs were transplanted into eight utricles, four saccules, and four organs of Corti. There was the integration of the implanted cells into three utricles and one saccule, but not in any of the organs of Corti. This result indicated that NSCs migrated and integrated into the sensory epithelium of the vestibular organs. In the study, all specimens in which NSCs integrated were exposed to a low concentration of gentamicin. NSCs did not integrate into organs that were severely damaged with a high concentration. The lack of cochlear NSC integration indicates that an improved method must be developed to combat the harsh cochlear environment [8].
In a different study performed in Sweden using NSCs to replace spiral ganglion neurons, adult mouse NSCs were transplanted into the mature guinea pig inner ears to investigate their survival and potential differentiation into auditory neurons. Spiral ganglion neurons (SGNs) are auditory neurons located in the Rosenthal’s spiral canal that runs inside the modiolus of the cochlea. Sound stimulation is transduced into an electrical signal in the mammalian organ of Corti’s hair cells, and then the spiral ganglion neurons receive this electrobiological signal [10]. To test whether it would efficiently derive a population of a specific neuronal type, one population of the cells were transduced with neurogenin 2, or ngn2, before inner ear transplantation. For the non-transduced adult, NSCs transplanted in normal-hearing guinea pigs implanted NSCs had low survival, and none remained 4 weeks after transplantation. Survival of NSCs was improved in animals that had been neomycin-treated (deafened) prior to transplantation. Similarly, improved survival rates were found in normal-hearing guinea pigs implanted with the ngn2-transduced NSCs. However, cell-counting revealed that the overall survival rate of the implanted adult NSCs was relatively low, possibly because the cochlear environment is not conducive to cell survival. Despite the low rate, this still demonstrates that adult stem cells can survive in the inner ear. The NSCs in the study also migrated from the implantation site in the scala tympani to locations along the auditory nerve tract close to the sensory epithelium and spiral ganglion neurons. As for neuronal differentiation, in normal-hearing hosts, there was none. In deafened animals, some surviving NSCs were labeled with a neuronal marker. In the animals implanted with ngn2-transduced NSCS, all animals with surviving NSCs had cells positively labeled with neuronal marker (TUJ1). This study demonstrated that NSCs can survive in the mature inner ear for up to four weeks after implantation, and that they have the ability to migrate to functionally important locations. In addition, it demonstrated that neuronal differentiation could occur with deafened animals as well as with transduced-NSCs [11].
A more recent study in China in 2016 investigated the restorative effects of olfactory epithelium neural stem cells (oe-NSCs) on noise-induced hearing loss in rat models. In the study, the implanted oe-NSCs were successfully able to ameliorate the hearing loss in the rats, as demonstrated by the improved auditory brainstem response (ABR) results. In addition, many GFP+ NSCs survived after implantation and migrated to the SGNs. This suggests that oe-NSCs restore hearing by ameliorating SGN condition. After incubation with 20 ng/mL of IL-1β, oe-NSCs displayed increased release of neurotrophic factors NGF and NT-3, which suggests that these cells function through upregulation of neurotrophic factor expression. Co-culturing the damaged neurons with the oe-NSCs also decreased the apoptotic rate of the damaged neurons from 45% in the controls to 15% with oe-NSCs [12]. Olfactory epithelium neural stem cells have also been shown to differentiate into hair cells. For instance, a study in Iran investigated the in vitro differentiation of NSCs isolated from rat olfactory epithelium. RT-PCR revealed that upon differentiation in a defined differentiation medium, the differentiated oe-NSCs displayed the hair cell-specific genes Math1, Myosin, Brn3, EPSIN, and AchRa9. Therefore, the study demonstrated that NSCs from the olfactory epithelium also present a potential source of cell therapy for hair cell regeneration [25].
Overall, further studies with NSCs will be required to increase the number of NSCs that survive and differentiate into spiral ganglion neurons and hair cells in the mature inner ear. Each of the studies showed that NSCs survive better in damaged cochleae and that the NSCs have the capability to migrate to functional areas. This indicates that NSCs are a possible source for cell replacement therapy for degenerative inner ear diseases that cause hearing loss [11].
Embryonic Stem Cell (ESC) Therapy
Embryonic stem cells are derived from the inner cell mass (ICM) of the pre-implantation blastocyst. ICM cells ultimately go on to form the embryo proper and thus have the ability to form all of the tissues of the body. Though ESCs are short-lived in vivo, they have the ability to be indefinitely propagated in vitro through growth in the presence of leukemia inhibitory factor (LIF) or on a feeder layer of murine embryonic fibroblasts (MEF) [13].
The first study performed with embryonic stem cells relating to the treatment of sensorineural hearing loss was in 2003. In this study, the research team used a stepwise approach involving growth factors to promote the differentiation of embryonic stem cells in vitro into inner ear progenitor cells, which eventually differentiated into mechanosensory hair cells. The team used chicken-mouse chimeras to demonstrate that ESC-derived inner ear progenitor cells could differentiate into hair cells in vivo. When positioned in a suitable environment, murine ESCs expressed hair cell marker proteins. While the success of the injection of murine ESCs into chicken inner ear sensory epithelia could have potentially been due to cell fusion of the murine cells and the chicken hair cells, this would be a rare event and would not explain the high efficacy of the experiment. This experiment demonstrates a protocol for generating inner ear progenitor cells in large numbers, which displays the promise of using ESC-derived progenitor cells in vivo in human patients to treat deafness [14].
In a 2014 study devoted to exploring the potential of human embryonic stem cells (hESCs) to differentiate into hair cells, a team demonstrated that hESCs could generate otic progenitor cells, whose growth depended on fibroblast growth factor (FGF) signaling. The team also showed that the ESC could express markers indicative of differentiated inner ear sensory epithelia. In addition, the differentiated hair cell-like cells expressed multiple hair cell markers simultaneously, and they displayed protrusions reminiscent of stereociliary bundles. However, the hESCs failed to fully mature into hair cells with the full hair cell cytoarchitecture. From these results, it can be determined that optimal conditions in vitro can be used to attain otic progenitors and thus sensory cell differentiation, but the procedure must be improved to attain the full hair cell structure [15].
A 2016 study demonstrated that ESCs can also be applied in the regeneration of SGNs. Stem cell therapies for generating auditory neurons to treat sensorineural hearing loss have been limited to animal models, which are not suitable for clinical applications. However, this study introduced a protocol to direct hESCs toward otic neuronal progenitors (ONPs) and SGNs. By treating the hESCs with human orthologs of ligands, small molecules, and neurotrophic factors, the team was able to generate a relatively pure population of cells that were phenotypically similar to SGNs. The team was able to successfully generate glutamatergic SGN-like cells, which could produce action potentials. To study neurite growth and connectivity, the team co-cultured the SGN-like cells with a rat brainstem slice. Although the cells extended some neurites to the NST, the cells extended more neurites to the CN (cochlear nucleus), and the pattern of transcription factor expression by the cells was similar to that of human SGNs. This ability to selectively control differentiation of ESCs is significant for the development of cell replacement therapy in SNHL [16]. A more optimal transplant procedure would be to generate three-dimensional ONP spheroids derived from ESCs, an aggregate form of ESC-derived ONP cells. Heuer, et. al demonstrated that these hESC-derived ONP spheroids are more conducive to survival and differentiation in transplantation by micropipette. In their study, the spheroids maintained characteristics similar to ONPs, which are precursors to SGNs, thereby demonstrating that this procedure is viable for transplantation of stem cells within the inner ear [26].
While it is clear from these studies that ESCs show great potential in their ability to replace hair cells and SGNs, researchers still face the hurdle of ensuring that the stem cells actually have the ability to restore hearing loss. In addition, there are ethical issues surrounding the use of ESCs, which are not present in alternative sources of stem cells. Legislation regarding the use of embryonic stem cells varies across the world, and in the U.S., federal funds can only be used on embryonic cell lines created before August 2001 with the rationale that these few lines do not compromise the sanctity of human life[7].
Mesenchymal Stem Cell (MSC) Therapy
In addition to hematopoietic stem cells, the bone marrow contains mesenchymal stem cells (MSCs), which have the ability to differentiate into cell types of all three embryonic germ layers [17]. MSCs can differentiate into chondrocytes, adipocytes, osteoblasts, and myocytes, as well as neurons both in vitro and in vivo [18]. Among the other types of stem cells, bone-marrow derived MSCs show great promise for cell replacement therapy in the inner ear. In a study performed in 2007, a research team led by Sang-Jun Jeon of Harvard Medical School used MSCs obtained from mouse bone marrow to generate sensory hair cells. Overexpression of the transcription factor Math1, which is necessary for the development of hair cells, induced the expression of hair cells markers that indicated differentiation of the MSC-derived progenitor cells into inner ear hair cells. Hair cell markers were also induced by culture of MSC-derived cells in contact with chick otocyst cells. After co-culture, there was increased expression of myosin VIIa, jagged2, p27Kip, Brn3c, and Math1. The positive results of this study indicate that MSCs are a viable choice for replacing damaged or lost hair cells when combined with the overexpression of Math1 [17].
In another 2015 study, a team applied the use of MSCs for SGN restoration. The team investigated the restorative effects of neural-induced human mesenchymal stem cells (NI-hMSCs) on sensory neuronal regeneration from neomycin-treated deaf guinea pig cochleae. Following in-vitro neural induction with basic fibroblast growth factor and forskolin, the hMSCs expressed high levels of neural markers, ionic channel markers, and tetrodotoxin-sensitive voltage-dependent sodium currents. This procedure demonstrated that hMSCs are capable of being differentiated into functional neural cells. After transplantation into the scala tympani of the damaged cochlea, NI-hMSC-injected guinea pigs displayed a large increase in the number of SGNs compared to the control. The transplanted cells migrated primarily around the spiral ganglion, organ of Corti, and cochlear nerve fiber in the damaged cochleae. In the spiral ganglion where the NI-hMSCs migrated, the implanted cells expressed the neuron-specific marker, NeuN. The results of this study indicate the potential of NI-hMSCs to replace damaged spiral ganglion neurons [18]. A more recent study in Korea found that MSCs could regenerate hair cells in an ototoxic deaf mouse model and therefore significantly improve hearing loss. A key result was that the ABR threshold of the MSC-group of mice was significantly higher than that of the control. Five weeks after transplantation of MSCs in the deaf mice, hair cell regeneration was confirmed from the basal turn to the apex of the cochlea in the MSC-group. Overall, the results of the study were that ototoxic hearing loss was successfully recovered by the injection of MSCs in the mice, and the Organ of Corti was restored by the MSC injection. These results indicate that MSCs have the ability to regenerate hair cells after damage [19].
Treatment of SNHL using MSCs has been tested in a human clinical trial as well. In a pilot study in Korea, the research team tested the safety and efficacy of trans-venously applying autologous bone marrow-derived MSCs to two SNHL patients. One patient had auditory neuropathy while the other did not. In both cases, the treatment produced no adverse side effects; however, there was no significant improvement of hearing for either patient when tested. There needs to be more testing to improve the transportation method for the MSCs in order to produce an improvement in auditory function in future trials [28]. In general, the findings of these studies demonstrate that MSCs have the potential to differentiate into inner ear hair cells and SGNs, but there is still more work to be done to improve the protocol and test more individuals.
Induced Pluripotent Stem Cell (iPSC) Therapy
Induced pluripotent stem cells (iPSCs) are formed by the forced expression of several transgenes, usually a mixture of Oct3/4, Sox2, Klf4, and c-Myc, which can reprogram human, mouse, rat, monkey, and dog somatic cells [20]. The iPSCs are highly similar to embryonic stem cells, but due to their somatic cell origin, they eliminate the ethical issues posed by the embryonic origin of ESCs. Human iPSCs (hiPSCs) can robustly proliferate in vitro and have the potential to differentiate into a variety of cell types of the body. Thus, this ability makes these cells a viable option for cell replacement therapy [21]. In a study performed at Stanford University School of Medicine, a research team used murine ESCs and iPSCs to generate a population of otic progenitor cells capable of differentiating into mechanosensory hair cells in vitro. The ESCs and iPSCs in the study generated otic progenitor cells, and they successful differentiated into hair cell marker-expressing cells. In certain cultures, these cells were also able to form cytomorphological specializations such as hair bundle-like protrusions. The cells responded to mechanical stimulation with currents that were reminiscent of immature hair cell transduction currents. In this study, there were no distinct differences between ESCs and iPSCs in terms of differentiation into hair cell-like cells. The results of the experiment demonstrate the possibility of using iPSCs, as well as ESCs, to generate mechanosensitive hair cell-like cells that could replace hair cells in cases of hearing disorders [22].
In another study performed in China, human urinary cells were reprogrammed to create iPSCs that were induced to differentiate into otic epithelial progenitors (OEPs) as well as hair cell-like cells, and then these cells were transplanted into mouse cochleae. The iPSCs in the study were induced to differentiate into the otic progenitor ONPs, capable of forming SGN-like cells, and OEPs, capable of forming hair cell-like cells. In vitro, OEP-derived hair cell-like cells were co-cultured with SGNs either dissected from the mouse or from the SGN-like cells derived from ONPs, the result of which was that the hair cell-like cells formed synaptic connections with the SGNs. After implantation in the mouse cochlea in vivo, some of the transplanted cells migrated to the organ of Corti, differentiated into hair cells, and formed synaptic connections with native SGNs in the cochlea. Therefore, it is reasonable to conclude from this study that transplantation of OEPs derived from iPSCs is feasible for regenerating lost hair cells [23].
Another study performed in China investigated whether mouse iPSCs could treat hearing loss in a SNHL mouse model by differentiating into hair cells and SGNs in the mouse cochlea. Four weeks after transplantation, CM-Di1-labeled iPSCs were found in the modiolus and Rosenthal’s canal. Some of the cells expressed hair cell markers or SGN markers in the group with transplanted iPSCs. As for the ABR threshold difference, the transplanted iPSCs slightly improved the ABR threshold, but there was not a significant difference between pre- and post-transplantation of iPSCs. While the transplanted iPSCs were able to migrate and differentiate into hair cell-like cells and SGN-like cells in the cochlea, the team failed to continue to observe the survival time and the final differentiation of the stem cells within the cochlea [24].
Induced-pluripotent stem cells pose the risk of introducing undesired genetic alterations, such as activation of oncogenes, in clinical applications. In order to reduce this risk, the reprogramming process must preserve genetic integrity of the induced cells while still enabling differentiation into the desired cell type. One study in the UK introduced reprogramming with synthetic mRNA as a safe way to reprogram hiPSCs for differentiation into otic progenitors and then hair cell and neuronal lineages. An important result of this study was that the hiPSC lines that were reprogrammed using nonintegrating mRNAs were able to differentiate into otic progenitors, as shown by the expression of otic markers PAX8, PAX2, FOXG1, and SOX2. Furthermore, these lines then successfully further differentiated into hair cell and neuronal lineages. The findings of this study indicate that mRNA-reprogrammed iPSCs are just as capable of producing otic lineages as lentivirus-induced ones while also being safer for clinical applications [27]. Overall, iPSCs in each study demonstrated that they are viable options for regenerating cells in the inner ear; however, more work still needs to be done to improve the survival and differentiation of iPSCs in the cochlea [24].
Conclusion
Based on the results of the studies, all four stem cell types demonstrate valuable potential for cell replacement therapy in treating sensorineural hearing loss. Each stem cell type demonstrated the ability to differentiate into both sensory hair cells and spiral ganglion neurons. For example, with NSCs, once transplanted in the cochlea, the implanted cells are able to migrate to functional locations along the auditory neuron and differentiate into SGNs [11].
Upon transplantation, the other stem cell types are also able to migrate to functional locations in the cochlea and differentiate into the necessary mature cell types. However, compared to the other types, ESCs face particular ethical issues that make them difficult to use for study [7]. Researchers have begun performing human clinical trials to investigate stem cell therapy for SNHL. Though there are currently few human clinical trials for stem cell therapy in SNHL patients, the clinical trial in Korea demonstrated that, with further testing, insertion of autologous bone marrow-derived MSCs may be a promising treatment for SNHL [28]. Despite the promising potential of these MSCs and other stem cells, much research is still needed to be done to improve the protocol for survival and differentiation of each stem cell type within the harsh environment of the human cochlea.
References
- Lin FR, Niparko JK, Ferrucci L. (2011) Hearing Loss Prevalence in the United States. Arch Intern Med. 171(20):1851-52.
- Michels TC, Duffy MT, Rogers DJ. (2019) Hearing Loss in Adults: Differential Diagnosis and Treatment. Am Fam Physician. 100(2):98-108.
- Szyfter W, Karlik M, Sekula A, Harris S, Gawecki W. (2019) Current indications for cochlear implantation in adults and children. OtolaryngologiaPolska = The Polish Otolaryngology. 73(3):1-5.
- Raman G, Lee J, Chung M, Gaylor JM, Sen S, et al. (2011) Effectiveness of Cochlear Implants in Adults with Sensorineural Hearing Loss. Agency for Healthcare Research and Quality (US).
- Oshima K, Grimm CM, Corrales CE, Senn P, Martinez Monedero R, et al. (2007) Differential distribution of stem cells in the auditory and vestibular organs of the inner ear. J Assoc Res Otolaryngol. 8(1):18-31.
- Reya T, Morrison SJ, Clarke MF, Weissman IL. (2001) Stem cells, cancer, and cancer stem cells. Nature. 414: 105-111.
- Alison MR, Poulsom R, Forbes S, Wright NA. (2002) An introduction to stem cells. J Pathol. 197(4):419-23.
- Fujino K, Kim TS, Nishida AT, Nakagawa T, Omori K, et al. (2004) Transplantation of neural stem cells into explants of rat inner ear. Acta Otolaryngol Suppl. (551): 31-3.
- Ito J, Kojima, K., & Kawaguchi, S. Survival of neural stem cells in the cochlea. Acta Otolaryngol. 2001; 121(2):140-2.
- Carricondo F, Romero-Gomez B. (2018) The Cochlear Spiral Ganglion Neurons: The Auditory Portion of the VIII Nerve. The Anatomical Record. 302(3):463-71.
- Hu Z, Wei D, Johansson CB, Holmstrom N, Duan M, et al. (2005) Survival and neural differentiation of adult neural stem cells transplanted into the mature inner ear. Exp Cell Res. 302(1):40-7.
- Xu YP, Shan XD, Liu YY, Pu Y, Wang CY, et al. (2016) Olfactory epithelium neural stem cell implantation restores noise-induced hearing loss in rats. NeurosciLett.616:19-25.
- Rippon HJ, Bishop AE. (2004) Embryonic stem cells. Cell Prolif. 37(1):23-34.
- Li H, Roblin, Liu H, Heller S. (2003) Generation of hair cells by stepwise differentiation of embryonic stem cells. ProcNatlAcadSci USA. 100(23):13495-500.
- Ronaghi M, Nasr M, Ealy M, Durruthy-Durruthy R, Waldhaus J, et al. (2014) Inner ear hair cell-like cells from human embryonic stem cells. Stem Cells Dev. 23(11):1275-84.
- Matsuoka AJ, Morrissey ZD, Zhang C, Homma K, Belmadani A, et al. (2017) Directed Differentiation of Human Embryonic Stem Cells Toward Placode-Derived Spiral Ganglion-Like Sensory Neurons. Stem Cells Transl Med. 6(3):923-36.
- Jeon SJ, Oshima K, Heller S, Edge AS. (2007) Bone marrow mesenchymal stem cells are progenitors in vitro for inner ear hair cells. Mol Cell Neurosci. 34(1):59-68.
- Jang S, Cho HH, Kim SH, Lee KH, Jun JY, et al. (2015) Neural-induced human mesenchymal stem cells promote cochlear cell regeneration in deaf Guinea pigs. ClinExpOtorhinolaryngol. 8(2): 83-91.
- Kim S, Kwon YH, Kim IB, Seo YJ, Han JS,et al. (2020) Therapeutic Efficacy of Bone Marrow Derived Mesenchymal Stem Cells in Ototoxic Sensorineural Hearing Loss. Korean J Otorhinolaryngol-Head Neck Surg. 63(12): 564-69.
- Okita K, Yamanaka S. (2011) Induced Pluripotent Stem Cells. Principles of Regenerative Medicine. 2011: 241-252.
- Yu J, Thomson JA. (2014) Induced Pluripotent Stem Cells. Principles of Tissue Engineering. 4:581-94.
- Oshima K, Shin K, Diensthuber M, Peng AW, Ricci AJ,et al. (2010) Mechanosensitive hair cell-like cells from embryonic and induced pluripotent stem cells. Cell. 141(4):704-16.
- Chen J, Hong F, Zhang C, Li L, Wang C, et al. (2018) Differentiation and transplantation ofhuman induced pluripotent stem cell-derived otic epithelial progenitorsin mouse cochlea. Stem Cell Res and Therapy. 9(230):1-15.
- Chen J, Guan L, Zhu H, Xiong S, Zeng L, et al. (2017) Transplantation of mouse-induced pluripotent stem cells into the cochlea for the treatment of sensorineural hearing loss. Acta Otolaryngol. 137(11):1136-42.
- Bahmani T, Gholami S, Mostafaie A, KarimiE, Hoseinkhani Z, et al. (2017) Olfactory Epithelium as an Infinitive Source of Neural Stem Cells for Derivation of Inner Ear Hair Cells. Intern J of Morphol. 35(2): 413-19.
- Heuer RA, Nella KT, ChangHT, Coots KS, Oleksijew AM, et al. (2021) Three-Dimensional Otic Neuronal Progenitor Spheroids Derived from Human Embryonic Stem Cells. Tissue Eng. 27(3-4):256-69.
- Boddy SL, Romero-Guevara R, Ji A, Unger C, Corns L, et al. (2020). Generation of Otic Lineages from Integration-Free Human-Induced Pluripotent Stem Cells Reprogrammed by mRNAs. Stem Cells Intern. 2020:1-10.
- Lee HS, Kim WJ, Gong JS, Park KH. (2018) Clinical Safety and Efficacy of Autologous Bone Marrow-Derived Mesenchymal Stem Cell Transplantation in Sensorineural Hearing Loss Patients. J Audiol Otol. 22(2):105-109.
This article was originally published in a special issue entitled “Stem Cells in Regenerative Medicine”, handled by Editor. Prof. Dr. Vincent S. Gallicchio.

