The MSH3 Gene from a Neuropsychiatrist’s Perspective
Adonis Sfera1*, Dan O Sfera1, Sarvin Sasannia2 and Sabine Hazan3
1Patton State Hospital; University of Southern California, Riverside, US
2Shiraz University of Medical Sciences, Shiraz, Iran
3Progenabiome.com
*Corresponding author: Sfera A, Patton State Hospital; University of Southern California, Riverside, US
Citation: Sfera A, Sfera DO, Sasannia S, Hazan S. (2022) The MSH3 Gene from a Neuropsychiatrist’s Perspective. Adv Clin Med Res. 3(4):1-6.
Received: September 29, 2022 | Published: October 20, 2022
Copyright© 2022 genesis pub by Sfera A, et al. CC BY-NC-ND 4.0 DEED. This is an open-access article distributedunder the terms of the Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International License.,This allows others distribute, remix, tweak, and build upon the work, even commercially, as long as they credit the authors for the original creation.
DOI: https://doi.org/10.52793/ACMR.2022.3(4)-40
Introduction
We read with great interest the article by Ambati BK, et al. “MSH3 Homology and Potential Recombination Link to SARS-CoV-2 Furin Cleavage Site” published on February 21st, 2022 in Frontiers in Virology. This perspective paper highlights a 19-nucleotide genetic sequence, a reverse complement of the human MSH3 gene, that contains the SARS-CoV-2 furin cleavage site (FCS). As this sequence (SEQ ID11652) was patented by Moderna in 2016 (US patent 9,587,003), some have suggested that the FCS may have been known prior to the COVID-19 pandemic [1].
Aside from its well-established role in averting tumor genesis, novel preclinical studies found that MSH3 is a key regulator of short tandem repeats (STRs), DNA sequences characteristic of monogenic neuropsychiatric disorders, such as Huntington’s disease (HD) and fragile X syndrome (FXS) [2-4]. For example, FXS is caused by CGG repeats in the fragile X messenger ribonucleoprotein 1 (FMR1) gene [5]. Interestingly, the designers of COVID-19 mRNA vaccine chose to encode the 42 arginine residues (found in viral S protein) via a rare CGG codon, increasing the odds of STRs formation [6]. Many viruses, including SARS-CoV-2, human cytomegalovirus (HCV), and human immunodeficiency virus (HIV), were demonstrated to generate STRs, increasing the risk of tandem repeat disorders (TRDs) [7-12]. On the other hand, fragile X mental retardation protein (FMRP), the product of FMR1 gene, was demonstrated to target influenza and Zika viruses, while metabotropic glutamate 5 receptor (mGluR5) inhibitors, commonly utilized in FXS, often ameliorate SARS-CoV-2 symptoms, indicating a two-way street between viral infections and TRDs [13-15].
Recent studies have reported that STRs can increase the risk of schizophrenia and autism spectrum disorder (ASD), indicating that these sequences play a major role not only in monogenic but also in polygenic disorders [16,17]. Indeed, the findings of Ambati BK, et al. are in line with our own studies that connected FCS to pathological cell-cell fusion, neurodegeneration, and psychopathology [18,19]. As COVID-19 mRNA vaccines elicit the expression of full-length S antigen (including the FCS), these therapeutics may promote pathological syncytia [20]. Along this line, giant cell myocarditis and arteritis due to pathological cell-cell fusion were recorded in Vaccine Adverse Event Reporting System (VAERS) [21,22].
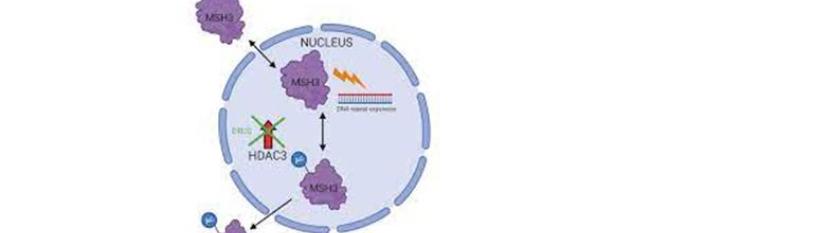
Pfizer and Moderna COVID-19 messenger RNA (mRNA) vaccines are heavily engineered to facilitate translation and improve stability, modifications that include codon optimization enriched with CG repeats [6]. However, as MSH3 regulates STRs, including the CG repetitions, vaccine efficacy is likely enhanced by the inhibition or attenuation of this protein. This may explain the reason Moderna was interested in patenting this molecule in 2016. In addition, as COVID-19 mRNA therapeutics encode the entire S antigen, MSH3may be over expressed, a phenomenon associated with loss of function [23]. Indeed, MSH3 may be inactivated via promoter methylation or over expression [24].
From the neuropsychiatric perspective, the novel MSH3 findings are significant as this protein, encoded on chromosome 5 (q11-q13), shares a common promoter with dihydrofolate reductase (DHFR), a gene disrupted in many neuropsychiatric conditions, including ASDs, schizophrenia, depressive and bipolar disorder as well as immune dysfunction, diabetes, type I, and epilepsy [25-31]. Due to the common promoter, vaccine-induced MSH3 inhibition likely attenuates DHFR, predisposing to these pathologies. In favor of this statement, we bring the fact that treatment with methotrexate, a DHFR inhibitor, was associated with neuropsychiatric pathology, including anxiety, depression, suicidal behavior, and dementia [16], [32-36].
Taken together, the MSH3/DHFR locus may represent a hub where immunity, metabolism, and neuropsychiatric pathology intersect, therefore a better understanding of these genes would shed light on the etiopathogenesis of these conditions.
Messenger RNA vaccines, a short overview
To elicit the generation of neutralizing antibodies, exogenously administered mRNA must be heavily engineered to avoid hydrolysis by the extracellular ARN se [37,38]. Placing the nucleic acid backbone into lipid nano particles (LNPs), hides it from RNAases, while replacing Uridine with N1-methylpseudouridine (m1Ψ), renders the vaccine undetectable to sensors [39,40] [Figure 1]. Other adjustments were made in the untranslated regions (UTRs) and polyadenylated (polyA) tail to protect and stabilize the vaccine [40,41]. Another alteration, addition of two proline residues, maintains the S antigen in prefusion conformation to enhance the exposure to host immune system [42]. Moreover, codon optimization includes increased CG content as well as G-quadruplex structures to promote quick translation [6]. Furthermore, MSH3 was also found to function as a sensor for G-quadruplexes, therefore opposing codon optimization [43,44].
Figure 1: N1-methylpseudouridine (m1Ψ)-modified mRNA (in the rectangle) is surrounded by a lipid nano particle (LNP) comprised of 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC), cholesterol, and an ionizable lipid. Polyethylene glycol (PEG) is conjugated with the lipid molecules to increase the mRNA duration of action. The mRNA encodes for the full-length S antigen and is flanked by two untranslated regions (UTRs) and a poly adenylated (poly) tail at the 3' end for stabilization. A cap at the 5’ end offers further protection from exonuclease recognition.
LNPs, a future prospect
LNP-incorporated mRNA comprises an enormous technological success that goes beyond the vaccines, opening new avenues for developing “smart” therapeutics that can be delivered with pinpoint precision to specific sub cellular structures [45]. The development of such therapeutics is anticipated to redefine clinical pathways, including for non-communicable diseases. However, are these therapies ready for worldwide application in their present molecular form? This question has been asked before, often in relation to the potential toxicity of lipid formulations used in the past, especially as part of cancer therapeutics delivery [46,47].
We anticipate that LNPs will be rapidly adopted into neuropsychiatry, especially as polyethylene glycol (PEG), an LNP component, can temporarily increase the permeability of blood-brain barrier (BBB), allowing direct nano particle access to neuronal networks [48]. Indeed, we envision a near future when micro or nano grams of LNP-attached psychotropic drugs could be delivered to intra neuronal targets, averting systemic adverse effects. However, for that to happen, the LNP may need to be redesigned as some of the currently utilized lipids may interfere with the psychotropic drugs as we recently documented [49].
References
- Ambati BK, Varshney A, Lundstrom K, Palú G, Uhal BD, et al. (2022) MSH3 Homology and Potential Recombination Link to SARS-CoV-2 Furin Cleavage Site. Front. Virol.
- Maksimov M, Ashbrook DG, BXD Sequencing Consortium, Villani F, Colonna V, et al. (2022) A novel quantitative trait locus implicates Msh3 in the propensity for genome-wide short tandem repeat expansions in mice bioRxiv.
- Hannan AJ. (2018) Tandem repeats mediating genetic plasticity in health and disease. Nat Rev Genet. 19(5):286-98.
- Crawford DC, Schwartz CE, Meadows KL, Newman JL, Taft LF, Gunter C, et al. (2000) Survey of the fragile X syndrome CGG repeat and the short-tandem-repeat and single-nucleotide-polymorphism haplotypes in an African American population. Am J Hum Genet. 66(2):480-93.
- Peprah E. (2012) Fragile X syndrome: the FMR1 CGG repeat distribution among world populations. Ann Hum Genet. 76(2):178-91.
- Xia X. (2021) Detailed Dissection and Critical Evaluation of the Pfizer/BioNTech and Moderna mRNA Vaccines. Vaccines (Basel). 9(7):734.
- Walker A, Petheram SJ, Ballard L, Murph JR, Demmler GJ, Bale JF Jr. (2001) Characterization of human cytomegalovirus strains by analysis of short tandem repeat polymorphisms. J Clin Microbiol. 39(6):2219-26.
- Savari H, Shafiey H, Savadi A, Saadati N, Naghibzadeh M. (2021) Statistics and Patterns of Occurrence of Simple Tandem Repeats in SARS-CoV-1 and SARS-CoV-2 Genomic Data. Data Brief. 36:107057.
- Laprevotte I, Pupin M, Coward E, Didier G, Terzian C, et al. (2001) HIV-1 and HIV-2 LTR nucleotide sequences: assessment of the alignment by N-block presentation, "retroviral signatures" of over repeated oligonucleotides, and a probable important role of scrambled stepwise duplications/deletions in molecular evolution. Mol Biol Evol. 18(7):1231-45.
- Keijzers G, Liu D, Rasmussen LJ. (2016) Exonuclease 1 and its versatile roles in DNA repair. Crit Rev Biochem Mol Biol. 51(6):440-51.
- Johnson BA, Xie X, Kalveram B, Lokugamage KG, Muruato A, et al. (2020) Furin Cleavage Site Is Key to SARS-CoV-2 Pathogenesis. bioRxiv.
- Villalba K, Devieux JG, Rosenberg R, Cadet JL. (2015) DRD2 and DRD4 genes related to cognitive deficits in HIV-infected adults who abuse alcohol. Behav Brain Funct. 11:25.
- Soto-Acosta R, Xie X, Shan C, Baker CK, Shi PY, et al. (2018) Fragile X mental retardation protein is a Zika virus restriction factor that is antagonized by subgenomic flaviviral RNA. Elife. 7:e39023.
- Zhou Z, Cao M, Guo Y, Zhao L, Wang J, et al. (2014) Fragile X mental retardation protein stimulates ribonucleoprotein assembly of influenza A virus. Nat Commun. 5:3259.
- Westmark CJ, Kiso M, Halfmann P, Westmark PR, Kawaoka Y. (2020) Repurposing Fragile X Drugs to Inhibit SARS-CoV-2 Viral Reproduction. Front Cell Dev Biol. 8:856.
- Mitra I, Huang B, Mousavi N, Ma N, Lamkin M, et al. (2021) Patterns of de novo tandem repeat mutations and their role in autism. Nature. 589(7841):246-50.
- Mojarad BA, Engchuan W, Trost B, Backstrom L, Yin Y, et al. (2022) Genome-wide tandem repeat expansions contribute to schizophrenia risk. Mol Psychiatry.
- Sfera A, Thomas KG, Andronescu CV, Jafri N, Sfera DO, et al. (2022) Bromodomains in Human-Immunodeficiency Virus-Associated Neurocognitive Disorders: A Model of Ferroptosis-Induced Neurodegeneration. Front Neurosci. 16:904816.
- Osorio C, Sfera A, Anton JJ, Thomas KG, Andronescu CV, et al. (2022) Virus-Induced Membrane Fusion in Neurodegenerative Disorders. Front Cell Infect Microbiol. 12:845580.
- Facciuolo A, Scruten E, Lipsit S, Lang A, Parker Cates Z, et al. (2022)High-resolution analysis of long-term serum antibodies in humans following convalescence of SARS-CoV-2 infection. Sci Rep. 12(1):9045.
- Cadiou S, Perdriger A, Ardois S, Albert JD, Berthoud O, et al. (2022) SARS-CoV-2, polymyalgia rheumatica and giant cell arteritis: COVID-19 vaccine shot as a trigger? Comment on: "Can SARS-CoV-2 trigger relapse of polymyalgia rheumatica?" by Manzo et al. Joint Bone Spine. Joint Bone Spine. 89(1):105282.
- Hirsch VG, Schallhorn S, Zwadlo C, Diekmann J, Länger F, et al. (2022) Giant cell myocarditis after first dose of BNT162b2 - a case report. Eur J Heart Fail. 24(7):1319-22.
- Marra G, Iaccarino I, Lettieri T, Roscilli G, Delmastro P, et al. (1998) Mismatch repair deficiency associated with overexpression of the MSH3 gene. Proc Natl Acad Sci U S A. 95(15):8568-73.
- Ni H, Jiang B, Zhou Z, Yuan X, Cao X, et al. (2017) Inactivation of MSH3 by promoter methylation correlates with primary tumor stage in nasopharyngeal carcinoma. Int J Mol Med. 40(3):673-78.
- Anderson S, Panka J, Rakobitsch R, Tyre K, Pulliam K. (2016) Anxiety and Methylenetetrahydrofolate Reductase Mutation Treated With S-Adenosyl Methionine and Methylated B Vitamins. Integr Med (Encinitas). 15(2):48-52.
- Roffman JL, Weiss AP, Purcell S, Caffalette CA, Freudenreich O, et al. (2008)Contribution of methylenetetrahydrofolate reductase (MTHFR) polymorphisms to negative symptoms in schizophrenia. Biol Psychiatry. 63(1):42-8.
- Drummond JT. (1999) Genomic amplification of the human DHFR/MSH3 locus remodels mismatch recognition and repair activities. Adv Enzyme Regul. 39:129-41.
- Frye RE, Rossignol DA, Scahill L, McDougle CJ, Huberman H, et al. (2020) Treatment of Folate Metabolism Abnormalities in Autism Spectrum Disorder. Semin Pediatr Neurol. 35:100835.
- Roffman JL, Petruzzi LJ, Tanner AS, Brown HE, Eryilmaz H, et al. (2018) Biochemical physiological and clinical effects of l-methylfolate in schizophrenia: a randomized controlled trial. Mol Psychiatry. 23(2):316-22.
- Flower M, Lomeikaite V, Ciosi M, Cumming S, Morales F, et al. (2019) MSH3 modifies somatic instability and disease severity in Huntington's and myotonic dystrophy type 1. Brain. 142(7):1876–86.
- Díaz-Casado E, Gómez-Nieto R, de Pereda JM, Muñoz LJ, Jara-Acevedo M,et al. (2020) Analysis of gene variants in the GASH/Sal model of epilepsy. PLoS One. 15(3):e0229953.
- Pinho de Oliveira Ribeiro N, Rafael de Mello Schier A, Ornelas AC, Pinho de Oliveira CM, Nardi AE, et al. (2013) Anxiety, depression and suicidal ideation in patients with rheumatoid arthritis in use of methotrexate, hydroxychloroquine, leflunomide and biological drugs. Compr Psychiatry. 54(8):1185-9.
- Newby D, Prieto-Alhambra D, Duarte-Salles T, Ansell D, Pedersen L, et al. (2020) Methotrexate and relative risk of dementia amongst patients with rheumatoid arthritis: a multi-national multi-database case-control study. Alzheimers Res Ther. 12(1):38.
- Sobel DO, Henzke A, Abbassi V. (2010) Cyclosporin and methotrexate therapy induces remission in type 1 diabetes mellitus. Acta Diabetol. 47(3):243-50.
- Frontera JA, Tamborska AA, Doheim MF, Garcia-Azorin D, Gezegen H, et al. (2022) Neurological Events Reported after COVID-19 Vaccines: An Analysis of VAERS. Ann Neurol. 91(6):756–71.
- Balasubramanian I, Faheem A, Padhy SK, Menon V. (2022) Psychiatric adverse reactions to COVID-19 vaccines: A rapid review of published case reports. Asian J Psychiatr. 71:103129.
- Andries O, Mc Cafferty S, De Smedt SC, Weiss R, Sanders NN, et al. (2015) N(1)-methylpseudouridine-incorporated mRNA outperforms pseudouridine-incorporated mRNA by providing enhanced protein expression and reduced immunogenicity in mammalian cell lines and mice. J Control Release. 217:337-44.
- Schoenmaker L, Witzigmann D, Kulkarni JA, et al. mRNAlipid nanoparticle COVID-19 vaccines: Structure an stability. Int J Pharm. 601:120586.
- Nance KD, Meier JL. ( Modifications in an Emergency: The Role of N1-Methylpseudouridine in COVID-19 Vaccines. ACS Cent Sci. 7(5):748-56.
- Gebre MS, Rauch S, Roth N, Yu J, Chandershekhar A, et al. (2022) Optimization of non-coding regions for a non-modified mRNA COVID-19 vaccine. Nature. 601(7893):410-14.
- Jeeva S, Kim KH, Shin CH, Wang BZ, Kang SM. (2021) An Update on mRNA-Based Viral Vaccines. Vaccines (Basel). 9(9):965.
- Riley TP, Chou HT, Hu R, Bzymek KP, Correia AR, et al. (2021) Enhancing the Prefusion Conformational Stability of SARS-CoV-2 Spike Protein Through Structure-Guided Design. Front Immunol. 12:660198.
- Flower M, Lomeikaite V, Ciosi M, Cumming S, Morales F, et al. (2019) MSH3 modifies somatic instability and disease severity in Huntington's and myotonic dystrophy type 1. Brain. 142(7):1876–86.
- Fleming AM, Burrows CJ. (2020) Interplay of Guanine Oxidation and G-Quadruplex Folding in Gene Promoters. J. Am. Chem. Soc. 142(3):1115-36.
- Pardi N, Hogan MJ, Porter FW, Weissman D. (2018) mRNA vaccines - a new era in vaccinology. Nat Rev Drug Discov. 17(4):261–79.
- Gokita K, Inoue J, Ishihara H, Kojima K, Inazawa J. (2020) Therapeutic Potential of LNP-Mediated Delivery of miR-634 for Cancer Therapy. Mol Ther Nucleic Acids. 19:330-38.
- Kulkarni JA, Cullis PR, van der Meel R. (2018) Lipid Nanoparticles Enabling Gene Therapies: From Concepts to Clinical Utility. Nucleic Acid Ther. 28(3):146-57.
- Rabanel JM, Piec PA, Landri S, Patten SA, Ramassamy C. (2020) Transport of PEGylated-PLA nanoparticles across a blood brain barrier model, entry into neuronal cells and in vivo brain bioavailability. J Control Release. 328:679-95.
- Sfera A, Hazan S, Anton JJ, Sfera DO, Andronescu CV, et al. (2022) Psychotropic drugs interaction with the lipid nanoparticle of COVID-19 mRNA therapeutics. Front Pharmacol. 13:995481.

