PYGO in Cancer Pathway
Shihori Tanabe*
Division of Risk Assessment, Center for Biological Safety and Research, National Institute of Health Sciences, Kawasaki 210-9501, Japan
*Corresponding author: Shihori Tanabe, Division of Risk Assessment, Center for Biological Safety and Research, National Institute of Health Sciences, Kawasaki 210-9501, Japan
Citation: Tanabe S. (2023) PYGO in Cancer Pathway. Adv Clin Med Res. 4(1):1-3.
Received: January 20, 2023 | Published: February 06, 2023
Copyright© 2023 genesis pub by Tanabe S. CC BY-NC-ND 4.0 DEED. This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International License.,This allows others distribute, remix, tweak, and build upon the work, even commercially, as long as they credit the authors for the original creation.
DOI: https://doi.org/10.52793/ACMR.2023.4(1)-48
Abstract
Several molecules are involved in cancer molecular network. Pygopus family plant homeodomain (PHD) finger (Pygo) is a component of Wnt/b-catenin transcription complex. Pygo has two homologs, Pygo1 and Pygo2, in mammalian cells. Pygo2 has an important role as a component of b-catenin - B-cell CLL/lymphoma 9 (Bcl9)-TCF/LEF complex. In this Editorial, a role of Pygo in Wnt/b-catenin signaling related to cancer pathway is summarized.
Introduction
What is PYGO?
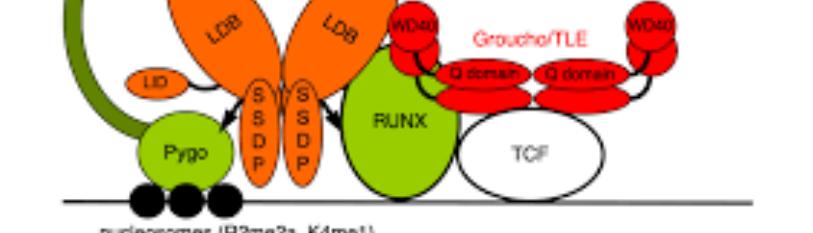
Pygopus family plant homology domain (PHD) finger (Pygo) is a dedicated component of the Wnt/b-catenin transcription complex [1]. Pygo is required for MYC, a basic helix-loop-helix leucine zipper protein, -dependent activation of mitosis-related genes and an essential component of MYC oncogenic activity [1]. Pygo has two homologs in mammalian cells, which are Pygo1, dispensable for normal murine development, and Pygo2, related to malignant growth in different cancers [1]. Pygo2 binds specific histone marks of activation such as H3K4me3, which promotes an open euchromatic structure as transcribing genes [1]. Pygo2 participates in the expression of highly transcribed RNAs essential for DNA replication and cell-cycle progression [1].
PYGO in Wnt/b-catenin signaling
Pygo is necessary for virtually all canonical Wnt signaling-dependent responses [2]. It has been demonstrated that mutations in B-cell CLL/lymphoma 9 (Bcl9) and Pygo genes result in congenital heart defects by tissue-specific perturbation of Wnt/b-catenin signaling in zebrafish [2]. The interaction between Pygo2 and di- and trimethylated lysine 4 of histone H3 (H3K4me2/3) is essential for mouse development and Wnt signaling-dependent transcription [3]. Pygo2 is more popular than Pygo1 in development, while Pygo1 and Pygo2 are considered to be tissue-specific Wnt pathway components [4]. Pygo2 is recruited by Bcl9 and Bcl9-like (Bcl9l) (Bcl9/9l) and sustains Pax6 expression to ensure a correct lens development in mice, independent of b-catenin [4].
PYGO in therapeutic-resistant cancer
It has been reported that the interactions of Bcl9/Bcl9L with b-catenin and Pygo promote breast cancer growth, invasion, and metastasis [5]. Bcl9/Bcl9L bind to Pygo and to the N-terminal domain of b-catenin via the homology domain 1 (HD1) and HD2 domains [5,6]. PYGO2 gene expression was down-regulated in diffuse-type gastric cancer compared to intestinal-type gastric cancer [7]. Diffuse-type gastric cancer demonstrates epithelial-mesenchymal transition-like phenotype which is related to therapeutic resistance in cancer [8]. Some correlations between PYGO2 expression and therapeutic-resistant cancer have been reported. Pygo promotes transcriptional activation of Wnt-target genes via b-catenin [6]. It may be possible that PYGO2 in Bcl9-TCF complex contributes to cancer progression in terms of Wnt/b-catenin pathway.
Conclusion
Pygo2 play a role in cancer pathway especially in correlation of Wnt/b-catenin pathway. PYGO2 expression seems to be associated with cancer phenotypes, whereas precise mechanism of the Pygo2-promoted therapeutic resistance in cancer is a way of the future.
References
- Andrews PGP, Popadiuk C, Belbin TJ, Kao KR. (2018) Augmentation of Myc-Dependent Mitotic Gene Expression by the Pygopus2 Chromatin Effector. Cell Rep. 23(5):1516-29.
- Cantu C, Felker A, Zimmerli D, Prummel KD, Cabello EM, et al. Mutations in Bcl9 and Pygo genes cause congenital heart defects by tissue-specific perturbation of Wnt/beta-catenin signaling. Genes Dev. 32(21-22):1443-58.
- Cantu C, Valenta T, Hausmann G, Vilain N, Aguet M, et al. The Pygo2-H3K4me2/3 interaction is dispensable for mouse development and Wnt signaling-dependent transcription. Development. 140(11):2377-86.
- Cantu C, Zimmerli D, Hausmann G, Valenta T, Moor A, et al. (2014) Pax6-dependent, but beta-catenin-independent, function of Bcl9 proteins in mouse lens development. Genes Dev. 28(17):1879-84.
- Vafaizadeh V, Buechel D, Rubinstein N, Kalathur RKR, Bazzani L, et al. (2021) The interactions of Bcl9/Bcl9L with beta-catenin and Pygopus promote breast cancer growth, invasion, and metastasis. Oncogene. 40(43):6195-09.
- Kramps T, Peter O, Brunner E, Nellen D, Froesch B, et al. (2002) Wingless Signaling Requires BCL9/Legless-Mediated Recruitment of Pygopus to the Nuclear β-Catenin-TCF Complex. Cell. 109(7):47-60.
- Tanabe S, Quader S, Ono R, Cabral H, Aoyagi K, et al. (2023) Regulation of Epithelial-Mesenchymal Transition Pathway and Artificial Intelligence-Based Modeling for Pathway Activity Prediction. Onco. 3(1):13-25.
- Tanabe S, Quader S, Ono R, Cabral H, Aoyagi K, et al. (2020) Molecular Network Profiling in Intestinal- and Diffuse-Type Gastric Cancer. Cancers. 12(12):3833.

