Treatment of Elbow Arthritis with a Bone Marrow derived Mesenchymal Stem Cell Extracellular Vesicle Isolate Product
John Bender and Maxwell Dordevic*
Addison Pain & Regenerative Medicine, 16633 Dallas Pkwy Suite 150, USA
*Corresponding author: Maxwell Dordevic, Addison Pain & Regenerative Medicine, 16633 Dallas Pkwy Suite 150, USA.
Citation: Bender J, Dordevic M. (2023)Treatment of Elbow Arthritis with a Bone Marrow derived Mesenchymal Stem Cell Extracellular Vesicle Isolate Product. J Orthop Study Sports Med.1(1):1-6.
Received: April 03, 2023 | Published: April 28, 2023
Copyright© 2023 genesis pub by Bender J. CC BY NC-ND 4.0 DEED. This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International License., This allows others distribute, remix, tweak, and build upon the work, even commercially, as long as they credit the authors for the original creation.
Abstract
This is a case study of a 60-year old active practicing chiropractor with an multi year history of an increasingly symptomatic traumatic osteoarthritic dominant elbow. She suffered a severe posterior elbow dislocation at the age of 16. Her symptoms were greatly exacerbated by work to the point she was considering retirement. Her surgical option was total elbow arthroplasty. She would be unable to return to her chiropractic practice following this surgical procedure. In an attempt to avoid surgery and continue to her chiropractic practice, she elected to try an elbow injection of the bone marrow-derived MSC EVIP containing active growth factors (over 800) and exosomes (over 10 billion per cc) (ExoFlo-Direct Biologics, St. Louis MO). Eighteen months following the injection her elbow is 90% improved, and she is working full time.
Keywords
Arthroplasty; Exosomes; Arthroscopic; Right elbow; Bone marrow; Primary osteoarthritis
Introduction
The elbow joint is a diarthrodial joint consisting of the ulno-humeral, ulno-radial and radial-humeral articulations. Primary osteoarthritis (OA) of the elbow is an uncommon condition associated with a genetic predisposition. Posttraumatic elbow OA is by far more common. Common traumatic insults to the elbow include soft tissue (subluxations and dislocations) and fractures of the humerus, ulna, radius or combinations. All of these may ultimately result in posttraumatic OA to the elbow. Elbow pain and/or loss of motion are the most common result of elbow arthritis from any etiology. Loss of extension and pronation can be compensated more easily than the loss of flexion and supination [1].
Nonoperative management of elbow arthritis typically includes elbow sleeves, nonsteroidal anti-inflammatory medications, and intra-articular corticosteroid injections. Avoidance of aggressive terminal flexion and extension activities can result in substantial relief of pain (weight-lifting, boxing, etc.). Physical therapy is reserved typically for patients presenting with an acute injury with an associated elbow joint effusion and limitations in motion [1].
A patient may be surgical candidate if they continue to have severe elbow pain or significant loss of mobility with resultant impairment of upper extremity function and limitation with daily activities even after a regimen of non-operative treatment. Advances in elbow arthroscopy have resulted in favorable outcomes and have totally replaced any open surgical debridement. Arthroscopic debridement of the elbow, particularly in a younger patient population, has reasonable results with improvements in pain and range of motion [2-5].It is important to note the published procedures are performed by surgeons with substantial experience with safe, meticulous techniques in elbow arthroscopy. Total elbow arthroplasty is Low-demand; elderly patient (> 60-year-old) with inflammatory, posttraumatic, or primary elbow arthritis may be a candidate for elbow arthroplasty. Severe elbow OA in a younger and typically male population may also be indicated [6].
There is a huge void between non-operative and operative treatment of elbow OA. This is a case report of a young, very active Chiropractor with OA of the dominant right elbow. She was treated with a single intra-articular injection of bone marrow-derived mesenchymal stem cell (MSC) Extracellular Vesicle Isolate Product (EVIP) containing active Growth Factors (over 800) and extracellular vesicles (EVs) over 10 billion per 2 cc.
Materials and Methods
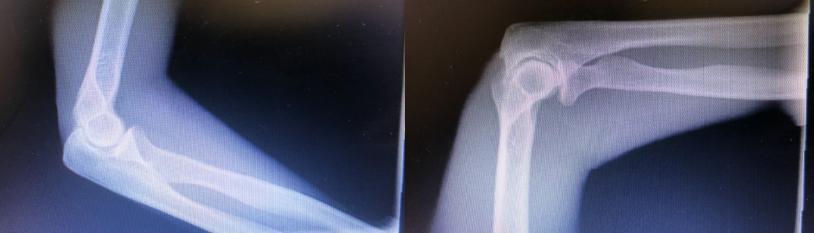
The patient is a very active, healthy 60-year old practicing Chiropractor. She initially injured her dominant right elbow at the age of 16 in a severe water-skiing accident. She suffered a complete dislocation of the elbow with her ulna and radius located posterior to the humerus. There was no neurovascular injury. The elbow was reduced under general anesthesia. After recovery, she lacked 150 of full extension and 100 of full flexion. There was no reduction in supination or pronation. She suffered a second injury at the age of 58, resulting in a complete radial head dislocation. Following this injury, she developed traumatic arthritis of the radiocapitellar joint with daily pain and joint swelling exacerbated with activities. Her ability to practice chiropractic care was severely limited. She regularly took NSAIDs and ice therapy after work. Her physical examination of the elbow revealed a 150 loss of elbow extension and 100 loss of flexion. She had a 100 loss of pronation and supination. There was one plus swelling of the elbow joint. Her radiographs reveal grade four Kellgren-Lawrence osteoarthritis of the elbow joint (Figure 1).
Figure 1: The left elbow radiograph is normal. The right elbow radiograph shows Kellgrin-Lawrence Grade 3 Osteoarthritis.
Elbow Injection
After counseling and consent, the patient underwent an elbow injection on 6/11/2019. The right elbow joint area was sterilized with a betadine skin prep. A 27-gauge needle was placed through a lateral and medial approach into the elbow joint. At this point, 2cc of the frozen EVIP (XoFlotm-Direct Biologics, St. Louis MO) was thawed to room temperature and placed into the medial and lateral side of the elbow joint. The patient experienced no immediate adverse reactions. The entire procedure took 15 minutes.
Results
Within 24 hours following the injection, the elbow joint became painful and swollen for several days. She was unable to work for a week. Her symptoms improved over the next four weeks. By six weeks post-injection, she felt her elbow was 50% better. This improvement has continued through 18 months and the elbow is now 90% improved from pre-injection. Elbow range of motion has returned to equal to the opposite elbow following the EVIP injection (Table 1) (Figure 2).
|
|
Pre-Injection |
6 Weeks |
3 Months |
6 Months |
18 Months |
Percent Improvement |
|
ODI |
26 |
12 |
6 |
4 |
2 |
92% |
|
UEFS |
24 |
42 |
54 |
72 |
76 |
68% |
|
BPI |
22 |
14 |
8 |
9 |
6 |
72% |
Table 1: Brief Pain Inventory (BPI), Upper Extremity Function Scale (UEFS), Oswestry Disability Index (ODI).
Figure 2: Increase in UEFS and decrease of ODI and BPI indicate patient improvement.
Discussion
This is a case study of a 60-year old active practicing chiropractor with a multi-year history of an increasingly symptomatic traumatic osteoarthritic dominant elbow. She suffered a severe posterior elbow dislocation at the age of 16. Her symptoms were greatly exacerbated by work to the point she was considering retirement. This was a significant decision because she is in a rural solo practice with financial overhead. Her surgical option was total elbow arthroplasty. She would be unable to return to her chiropractic practice following this surgical procedure. The goal of the surgery was to attempt to improve her activities of daily living, not a return to strenuous work. In an attempt to avoid surgery and continue to practice chiropractic, she elected to try an elbow injection of the bone marrow-derived MSC EVIP containing active growth factors (over 800) and over 10 billion per cc of extracellular vesicles (XoFlo-Direct Biologics, St. Louis MO). Eighteen months following the injection her elbow is 90% improved, and she is working full time.
The elbow is a di-arthrodial joint with a synovial lining and a joint capsule. There are more synovial capsular MSCs than are found in bone marrow or adipose tissue. These MSCs have more chondrogenic potential than bone or adipose MSCs [7]. During the development of OA, pro-inflammatory growth factors are produced by these synovial MSCs. This contributes to a chronically inflamed, painful joint environment. Bone marrow concentrate (BMC) contains on average only about 2,500 MSCs per cc [8].Despite the incredibly small number of MSCs found in BMC, there are literature reports of clinical efficacy in animals and humans using BMC for the treatment of OA [9-11].This effect may not be dependent upon BMC/MSC cell survival or differentiation. The efficacious effect may be from the release of acellular paracrine factors. The utilization of acellular MSC derived growth factors and especially EVs may be the future of the biologic treatment of OA. The extracellular vesicle is a bi-phospholipid membrane-enclosed structure created by the intracellular organelle the endosome. It is typically the size of 30 to 150 nanometer (1 billionth of a meter). One thousand times larger is the actual MSC ranging in size from 12 to 18 microns (1 Million of a meter). EVs contain growth factors, signaling lipids and micro, and messenger RNA. The RNA contents in EVs mediate most of their anti-inflammatory effects. EVs can be placed into any joint in concentrations of 100,000 or more times that of any cellular MSC treatment. These growth factor proteins and EVs are believed to function in a paracrine fashion to, directly and indirectly, alter the inflammatory environment of any painful arthritic joint back to a normal non-painful physiologic environment [12-15].
The acellular EV treatment for OA will involve a two-front attack involving anti-inflammatory growth factors and RNAs into the arthritic joint. These EVIP growth factors will enter the nucleus of the recipient synovial MSC and stimulate the transcription of mRNA containing instructions for the production of continuous anti-inflammatory secretomes, chemokines, and cytokines. These will be released from the recipient synovial MSC into the synovial fluid. Second, the highly concentrated EVs from the EVIP will enter recipient synovial MSCs to deliver their mRNA. Once delivered, mRNA will directly undergo translation in the recipient synovial MSC ribosomes to produce anti-inflammatory secretomes, cytokines, and chemokines. These intra-articular anti-inflammatory effects could continue for months or years. This acellular biologic treatment can all be achieved with a single arthritic joint injection, not requiring the morbidity and cost of obtaining autogenous MSCs. The future of regenerative medicine in orthopedics and spine may well be the utilization of highly concentrated acellular MSC bone marrow-derived growth factors and especially extracellular vesicles [12-16].
References
- Biswas D, Wysocki RW, Cohen MS. (2013) Primary and Secondary Arthritis of the Elbow. Arthritis. 2013:473259.
- Savoie FH, Nunley PD, Field LD. (1999) Arthroscopic management of the arthritic elbow: indications, technique, and results. J Shoulder Elbow Surg. 8(3):214-9.
- Ball CM, Meunier M, Galatz LM, Calfee R, Yamaguchi K. (2002) Arthroscopic treatment of post-traumatic elbow contracture. J Shoulder Elbow Surg. 11(6):624-29
- Nguyen D, Proper SIW, MacDermid JC, King GJW, Faber KJ. (2006) Functional outcomes of arthroscopic capsular release of the elbow. Arthroscopy. 22(8):842-49.
- Kelly EW, Bryce R, Coghlan J, Bell S. (2007) Arthroscopic debridement without radial head excision of the osteoarthritic elbow. Arthroscopy. 23(2):151-56.
- Little CP, Graham AJ, Carr AJ. (2005) Total elbow arthroplasty: a systematic review of the literature in the English language until the end of 2003. J Bone Joint Surg. 87(4):437-44
- Chang CH, Huo TF, Lin FH, Wang JH, Hsu YM, et al. Tissue engineering-based cartilage repair with mesenchymal stem cells in a porcine model. J Orthop Res. 29:1874-80.
- Pettine KA, Murphy MB, Suzuki RK, Sand TT (2015) Percutaneous injection of Autologous bone marrow concentrate significantly reduces lumbar discogenic pain through twelve months. Stem Cells. 33:146-156.
- Black LL, Gaynor J, Adams C, Dhupa S, Sams AE, et al. (2008) Effect of intraarticular injection of autologous adipose-derived mesenchymal stem and regenerative cells on clinical signs of chronic osteoarthritis of the elbow joint in dogs. Vet Ther. 9(3):192-200.
- Guerico A, Di Marco P, Casella S, Cannella V, Russotto L, et al. (2012) Production of canine mesenchymal stem cells from adipose tissue and their application in dogs with chronic osteoarthritis of the humeroradial joints. Cell Biol Int. 36(2):189-94.
- Mokbel A, El-Tookhy O, Shamaa AA, Sabry D, Rashed L, et al. (2011) Homing and efficacy of intra-articular injection of autologous mesenchymal stem cells in experimental chondral defects in dogs. ClinExpRheumatol. 29(2):275-8.
- Li Z, Wang Y, Xiao K, Weng X. (2018) Emerging Role of Exosomes in the Joint Diseases. Cell PhysiolBiochem. 47(5):2008-2017.
- Chang Y, Wu K, Harn H. (2018) Exosomes and Stem Cells in Degenerative Disease Diagnosis and Therapy. Cell Transplant. 27(3):349-63.
- Cheng L, Zhang K, Wu S, Cui M, Xu T. (2017) Focus on Mesenchymal Stem Cell-Derived Exosomes: Opportunities and Challenges in Cell-Free Therapy. Stem Cells Int. 2017:63052
- Zhang S, Chuah SJ, Lai RC, Hui JHP, Lim SK, et al. MSC Exosomes Mediate Cartilage Repair by Enhancing Proliferation, Attenuating Apoptosis and Modulating Immune Reactivity. Biomaterials. 156:16-27.
- Kellgren J, Lawrence J. (1957) Radiological assessment of Osteo-Arthrosis. Ann Rheum Dis. 16(4):494-502.

