Guillain-Barre Syndrome Mistaken for a Lumbar Spinal Disorder: A Case Report
Dinh Thi Phuong Hoai*, Mai Dang Thi, Pham Hai Duong, Tran Nhat, Tran Thi Mai Dieu, Nguyen Duy Duan, Nguyen Vinh Lac, Nguyen Thanh Minh
Hue University of Medicine and Pharmacy, Hue University, Vietnam
*Corresponding author: Dinh Thi Phuong Hoai, Hue University of Medicine and Pharmacy, Hue University, Vietnam.
Citation: Hoai DTP, Thi MD, Duong PH, Nhat T, Dieu TTM, et al. (2021) Guillain-Barre Syndrome Mistaken for a Lumbar Spinal Disorder: A Case Report. J Neurol Sci Res. 2(1):1-08.
Received: October 20, 2021 | Published: November 03, 2021
Copyright ©️ 2021 genesis pub by Hoai DTP, et al. CC BY-NC-ND 4.0 DEED. This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-No Derivatives 4.0 International License. This allows others distribute, remix, tweak, and build upon the work, even commercially, as long as they credit the authors for the original creation.
DOI: http://doi.org/10.52793/JNSR.2021.1(2)-09
Abstract
Guillain-Barre syndrome (GBS) is an inflammatory demyelinating polyneuropathy. In typical cases, GBS often presents with discomfort, numbness, paresthesia, and weakness in the limbs. Autonomic involvement is common leading to urinary retention and ileus. Many of these signs are correlated with those of spinal lumbar disorder. It can therefore be difficult, particularly early in the course of GBS, to correctly diagnose GBS in a patient with symptomatic lumbar spinal disorder or a patient with atypical GBS manifestations. Here, we report on a case of atypical GBS in a previously healthy 30-year-old man previously healthy patient with lumbar disc herniation and Tarlov cyst at S1-S2 level, and discuss the differential diagnosis of the GBS and lumbar spinal disorder. The aim of the study was to investigate a case of Guillain-Barre syndrome (GBS) in which the patient presented with back pain, leg pain, and weakness that caused the initial diagnostic error.
Keywords
Guillain barre syndrome; Inflammatory demyelinating polyneuropathy; Lumbar disc herniation; Tarlov cyst
Introduction
Guillain-Barré syndrome (GBS) is an acute inflammatory demyelinating polyneuropathy and is a significant cause of acute neuromuscular paralysis, especially with the almost complete eradication of poliomyelitis. Its incidence ranges from 0.8 to 1.9 cases per 100,000 people in North America and Europe. GBS affects people of all races and ages, but is more prevalent in topics over 50 years of age and for every 10-year age increase, the incidence rises by 20% [1-3]. The annual GBS incidence increased from 1.7/100,000 to 3.3/100,000 after 50 years [1].
The first signs of GBS in typical cases are discomfort, numbness, paresthesia, and weakness in the limbs. Initially, the deficiency can be proximal, distal or a mix of both and rapidly progressive [4]. The extremities are normally affected with numbness and paresthesia and distributed proximally. On examination, decreased tendon reflexes or areflexia are observed. The diagnosis is generally clear [5]. However, the diagnosis of GBS is difficult in patients with asymmetrical weakness, in those with weakness only in the lower extremities or in those with symptomatic disc herniation.
We concentrate on the diagnosis of GBS and the increasing clinical spectrum, the frequent incidence of pain and autonomic dysfunction, and recent insights into the syndrome's pathogenesis. Furthermore, prognostic modeling and current treatment options available during GBS are discussed. The purpose of this review is to combine the latest laboratory and clinical developments that may lead to improved care options for GBS patients.
A Case Report
A 30-year-old man presented to the emergency department with low back pain, bilateral numbness and weakness in the lower extremities. His symptoms had started one day prior, after carrying a heavy box and got worse. He had no history of recent vaccination or infectious diseases. Sometimes, he had back problems and got treatment with medication. Physical examination revealed weakness of Medical Research Council (MRC) Muscle Scale 4/5 in the left lower extremity and 4+/5 in the right lower extremity. Numbness was not correlated with dermatomal distribution in the lower extremities. The sensory function was normal in the lower extremities. Deep tendon reflexes have all been hypoactive, including biceps jerk, knee jerk and ankle jerk, the Babinski’s sign and Lasègue’s sign were negative. Gait could not be evaluated due to the weakness of the muscles.
Investigations: Leukocytes 5.73 K/µL, hemoglobin 16.2 g/dL, hematocrit 46.7%, platelets 336 K/µL, serum glucose 99 mg/dL, urea 5.74 mmol/L, creatinine 71.3 mmol/L.
Electromyography
Motor nerves: Long-lasting latent motor potential of the tibial nerve and the fibula, reducing the speed of the tibial nerve conduction and the deep fibrous nerve (Table 1).
|
Result |
Lat.[ms] |
Ampl[mV] |
NCS[m/s] |
Dist[cm] |
Stim[mA] |
|
Fibularis right |
6.8 |
6.8 |
- |
- |
55 |
|
Fibularis right |
15 |
6.4 |
42.7 |
35 |
55 |
|
Tibial right |
11.6 |
10.3 |
- |
- |
40 |
|
Tibial right |
22.1 |
7.6 |
34.8 |
36.5 |
40 |
|
Fibularis left |
9.6 |
3.3 |
- |
- |
50 |
|
Fibularis left |
18.5 |
3.1 |
38.2 |
34 |
50 |
|
Tibial left |
12.1 |
10.5 |
- |
- |
40 |
|
Tibial left |
23.7 |
7.2 |
32.8 |
38 |
40 |
Table 1: Motor nerves; Long-lasting latent motor potential of the tibial nerve and the fibula, reducing the speed of the tibial nerve conduction and the deep fibularis nerve.
Sensory nerves: reduce the rate of nerve conduction of both sides. F wave and H reflex: H reflex loss, F wave elongated lower limb nerve (Table 2).
|
Result |
F-min[ms] |
F-max[ms] |
F-mean[ms] |
F-var[ms] |
Pers[%] |
|
Tibial right |
57.1 |
57.1 |
57.1 |
0 |
10 |
|
Tibial left |
60.4 |
60.4 |
60.4 |
0 |
10 |
Table 2: Sensory nerves; reduce the rate of nerve conduction of both sides. F wave and H reflex: H reflex loss, F wave elongated lower limb nerve.
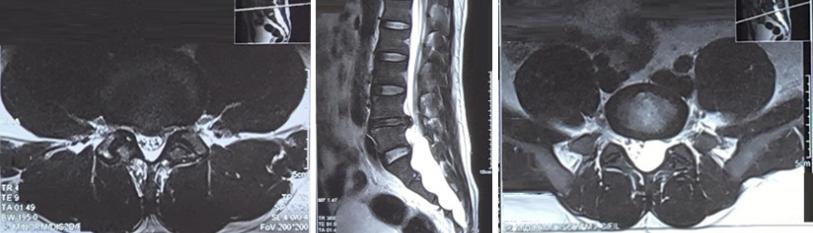
CSF cytochemistry: very scarce erythrocytes, 99% normal, 1% crenate, yeasts was negative, leukocytes were not observed, glucose 4.51 mmol/L, proteins 1.42g/L, Pandy reaction is positive. Magnetic resonance imaging (MRI) of the lumbar spine revealed disc protrusion at the L4-L5 level and Tarlov cyst at S1-S2 level with the size of 4.5x2.5x11 cm compressing the cauda equina (Figure 1). The patient had plasma exchanged, plasma exchange had begun for four days with an exchange of approximately three liters of plasma per day. Ten days after the onset of symptoms, his weakness began to improve and, eleven days after the onset of weakness, he was able to walk with assistance. One month after the onset of symptoms, with no numbness, his pain and fatigue improved a great deal. He could walk without assistance but limping and numbness on the lower extremities remained.
Figure 1: MRI of the lumbar spine; disc protrusion at the L4-L5 level (Left) and Tarlov cyst at S1-S2 level with size 4.5×2.5×11cm compressed the cauda equina (Middle and Right).
Discussion
The major cause of neuromuscular paralysis is Guillain Barré syndrome (GBS), and it has been mentioned worldwide. GBS has been considered a single disease for several years since its initial diagnosis and is synonymously referred to as acute inflammatory demyelinating polyradiculoneuropathy (AIDP), based on evidence of acute myelin immune attack and similarity to experimental allergic neuritis. Acute Inflammatory Demyelinating Polyradiculoneuropathy (AIDP) is the prototype of GBS and described as peripheral nerve and spinal root demyelination. Up to 90% of GBS cases in North America and Europe account for this disorder [6] but only 22-46% of the cases in China, Japan, India, Southeast Asia, and Mexico [7]. This disorder is characterized by myelin vesicular degeneration caused by the complex development of membrane-attack Ig on the outer surface of Schwann cells. There was no clear connection between AIDP and the antiganglioside antibodies [8].
More recently, it has become clear that AIDP depicts only the GBS prototype, and other associated immune polyneuropathies have been grouped and named GBS that cause immediate generalized weakness but with different etiologies and pathophysiologies. These forms of GBS most often include axonal subtypes of GBS, in which axonal degeneration and non-segmental demyelination are the main pathology, including acute motor axonal neuropathy (AMAN), pure motor disorder, and acute motor sensory axonal neuropathy (AMSAN) or acute mixed sensor motor axonopathy (AMSAN) [7].
Clinical Features
GBS is primarily motor neuropathy in its normal form, while sacral paresthesia is almost always present at the onset of the disease. Feelings of tingling, prickling, or pins and needles are usually accompanied by symmetrical leg weakness and difficulty walking within hours or days. The presence of sacral paresthesia increases the possibility of proper GBS diagnosis [9]. It is common to have trouble ascending stairs or emerging from a chair or toilet seat. Distal weakness is more common but proximal weakness is more severe than distal weakness. Sometimes, the weakness spreads to the upper limbs, leading to ascending paralysis. If the disease progresses, there may be weakness that remains exclusive in the legs or, alternatively, weakness that occurs in the hands or shoulder girdle and involves the legs. Approximately 70% of patients have no deep tendon reflexes when they are tested for the first time. Up until fatigue or broad fiber sensory loss progresses, reflexes occasionally remain elicitable. In limbs that are too frail to against gravity, reflexes are almost always unobtainable. Most patients complain of sensations of "pins and needles," "prickling," or "tingling," along with a "asleep feeling" in an arm or leg after limb compression. In comparison to other length-dependent axonal polyneuropathies, paresthesia in the fingertips sometimes occurs in patients with GBS shortly after the foot is affected, and often beforehand. As the disease progresses, the sensory signs are symmetrical and frequently precede fatigue by a few days, before ascending to the ankles and wrists. A sacral numb, heavy, or dead feeling can also be identified by patients as the illness progresses. Some experience sensory impairment over the trunk and have identified a well-defined sensory level simulating spinal cord disease, but only to the point of a significant shift in sensation, not below-level analgesia.
A common but unappreciated symptom of GBS is pain. Pain in 1/3 of the patients can accompany the onset of weakness by 2 weeks [10], Around 2/3 of the patients have modest pain early in the disease [11]. GBS dissatisfaction has been defined as [1] pain, typically limited to the back, hips, or upper leg muscles (the most common type); [2] shooting or stabbing, radicular pain radiating from the back to one or both legs; or [3] chronic and unrelenting, burning, dysesthetic feelings in the distal limbs [10-12].
Rarely, back and radicular pain can precede weakness and paresthesias and thus be attributed to sciatica or a spinal condition [12].
In around 20-30 % of patients with GBS, the weakening of the diaphragm that contributes to respiratory failure and a need for ventilation support occur. Following 2 weeks into the course of the disease, if diaphragmatic and respiratory muscle weakness has not occurred, assisted ventilation should not be necessary unless other pulmonary or medical problems result [7].
Diagnosis
GBS remains a comprehensive diagnosis of a disorder for which no specific diagnostic tests are available [5]. For standard GBS, diagnostic criteria such as: Weakness can affect all limb muscles equally, or distal or proximal muscles in the arms or legs primarily. At least in the affected limbs, patients have reduced or absent deep-tendon reflexes. In patients suspected of having GBS, a lumbar puncture is almost always performed. Usually, CSF testing indicates elevated protein with a normal white cell count of CSF. NCS and EMG play a very important role in the diagnosis, classification of subtypes and confirmation of peripheral neuropathy [4,13]. Abnormalities in nerve conduction studies (NCS) are seen in up to 95% of cases, and these findings are seen at various points during the course of the disease in a significant number of GBS patients [14]. In these cases, retesting in 1 to 2 weeks might be required to confirm the diagnosis [4,13]. It is important to thoroughly evaluate multiple nerves and multiple nerve segments in multiple limbs, including the assessment of F-waves, H-reflexes, and blink reflexes. NCS seeks to demonstrate evidence of acquired multifocal nerve demyelination, the hallmarks of AIDP, which accounts for the majority of GBS patients in the Western World [6]. F wave and H reflex in this patient: H reflex loss, F wave, elongated lower limb nerve. The protein level in Cerebrospinal Studies Fluid (CSF) is elevated in most patients at any points during the course of the disease’s course. Elevated CSF protein concentration presents in only 50% of patients during the first week of illness, and this number rises to 75% by the third week [9]. The cause of elevated CSF protein is not known but it is likely resulting from blood-CSF barrier abnormalities due to inflammation at the level of the roots of the spinal nerve roots.
Spinal MRI is a responsive diagnostic test suggested by some authors and should be considered as an additional diagnostic method and helpful in excluding central nervous system disorders that may resemble GBS in their presentations. Gadolinium spinal MRI also shows the improvement of the spinal nerve roots early in the course of pediatric GBS and lets clinicians develop a diagnosis early in the course of the disease while other diagnostic tests may still yield normal results [15]. Lumbar spine MRI is frequently abnormal and illustrates cauda equina nerve root stimulation with gadolinium. This happens in up to 80-90% of patients, particularly in children and patients with serious weakness and severe leg and back pain [7]. These signs and symptoms are typically bilateral but may be asymmetric [16]. It can be difficult to diagnose GBS, particularly in patients with pre-existing symptomatic disc herniation of the disc and a narrow spinal canal resulting from a large herniated disc. In the present case, the cauda equina was compressed by disc herniation at the level of L4-L5 and by Tarlov cyst at S1-S2 level with a size of 4.5×2.5×11 cm. We were worried about pain, about progressive paraparesis. But in comparison, rapidly progressive paraparesis was presented in the patient and this was more suggestive of GBS rather than disc herniation and Tarlov cyst.
Treatment
Therapeutic plasma exchange (PE) used as a treatment modality in many autoimmune disorders, including neurological conditions, such as Guillain-Barré syndrome (GBS) and chronic inflammatory demyelinating polyradiculoneuropathy (CIDP). Based on published evidence to help physicians in both the medical and technological aspects of apheresis consultation, the American Society for Apheresis (ASFA) publishes its recommendations on the use of therapeutic apheresis every 3 years. The ASFA guidelines included the use of PE as an acceptable first line therapy in both GBS and CIDP, either alone and/or in combination with other treatment methods. The role of apheresis in these conditions and different technical aspects of the PE procedure were discussed, such as the measurement of apheresis, the number of volume exchanges, the replacement fluid and the treatment of possible complications [17].
When being used within the first 4 weeks of onset, PE was beneficial, but when they tried starting it earlier (i.e, within the first 2 weeks), the greatest effect was seen [18,19]. In addition, the North American PE study was the first major trial to demonstrate a beneficial impact of immunotherapy on GBS [18]. After the publication of these findings, IVIg for 5 consecutive days in the 0.4 g/kg bodyweight daily regimen has replaced PE as the preferred treatment in many centers for 5 consecutive days in the 0.4 g/kg bodyweight daily regimen, primarily because of its greater convenience and availability. The Cochrane review on the use of IVIg in GBS contained four additional trials [20]. There was no difference between IVIg and PE in terms of change in the grade of impairment after 4 weeks, mechanical ventilation duration, mortality or residual disability.
In our case, a Tarlov cyst, which was initially recorded on the MRI, could easily be the radiographic characteristics and position of the sacral canal fluid-intensity lesion. Tarlov cysts are meningeal dilations of nerve roots filled with cerebrospinal fluid, most commonly located in the spinal canal of the spinal cord section of S1-S5 [21]. The etiology of Tarlov cysts was not known well, although many theories that could be either congenital or acquired have been suggested. Congenital causes include connective tissue diseases such as Loeys Dietz, Ehlers-Danlos, and Marfan syndrome, while acquired causes include nerve root cyst inflammation, arachnoidal expansion around and around the exiting nerve root, haemorrhagic spinal tissue accumulation, and perineurium and epineurium venous drainage breakage secondary to haemosiderin deposition after trauma [22]. Tarlov cysts may cause back pain, sciatica, perineal, buttock and lower extremity pain as well as genital, urinary and bowel dysfunction, but MRI is often neglected or thought to be of little value in developing back pain [23]. Our patient: manifestations of lower limb weakness being suspected of acute cauda equina syndrome lesion, MRI of the lumbar spine was assigned, and Tarlov cyst of 4.5×2.5×11cm was incidentally found. The most popular methods of diagnosing Tarlov cysts are lumbosacral MRI and CT myelography, but dedicated sacral MRI is more sensitive [23]. Treatment options include non-surgical drainage of lumbar cerebrospinal fluid and percutaneous drainage of cysts, and other surgical options such as cyst fenestration, resection of the cyst wall, quick decompressive laminectomy and reconstruction and closure of myofascial flaps. More recently, less invasive techniques have been identified for the treatment of Tarlov cysts, including the two-needle method used in this case. Lidocaine or Marcaine was inserted into the cysts prior to this procedure. This is a practical examination to assess whether cysts are symptomatic [22].
Conclusion
There are possibilities in confusing atypical symptoms and signs of GBS with those of a lumbar spinal disorder due to the lacking of tests that are highly sensitive, especially early in the course of the disease. For proper treatment and to prevent unnecessary surgery for lumbar spinal stenosis, close monitoring of the clinical course and comprehension and suspicion of the possibility of GBS are important.
References
- McGrogan A, Madle GC, Seaman HE, de Vries CS. (2009) The epidemiology of Guillain-Barré syndrome worldwide. A systematic literature review. Neuroepidemiology. 32(2):150-63.
- Van der Maas NA, Kramer MA, Jacobs BC, van Soest EM, Dieleman JP, et al. (2011) Guillain-Barré syndrome: background incidence rates in The Netherlands. JPNS. 16(3):243-9.
- Sejvar JJ, Baughman AL, Wise M, Morgan OW. (2011) Population incidence of Guillain-Barré syndrome: a systematic review and meta-analysis. Neuroepidemiol. 36(2):123-33.
- Hughes RA, Cornblath DR. (2005) Guillain-Barré syndrome. Lancet. 366(9497):1653-66.
- van Doorn PA, Ruts L, Jacobs BC. (2008) Clinical features, pathogenesis, and treatment of Guillain-Barré syndrome. The Lancet Neurol. 7(10):939-50.
- Hadden RD, Cornblath DR, Hughes RA, Zielasek J, Hartung HP, et al. (1998) Electrophysiological classification of Guillain-Barré syndrome: clinical associations and outcome. Plasma Exchange/Sandoglobulin Guillain-Barré Syndrome Trial Group. Ann Neurol. 44(5):780-8.
- Piccione EA, Salame K, Katirji B. (2014) Guillain-Barré Syndrome and Related Disorders. In: Katirji B, Kaminski HJ, Ruff RL, editors. Neuromuscular Disorders in Clinical Practice. New York, NY: Springer New York; pp. 573-603.
- Hiraga A, Kuwabara S, Ogawara K, Misawa S, Kanesaka T, et al. (2005) Patterns and serial changes in electrodiagnostic abnormalities of axonal Guillain-Barré syndrome. Neurol. 64(5):856-60.
- Yuki N, Hartung HP. (2012) Guillain-Barré syndrome. NEJM. 366(24):2294-304.
- Ruts L, Drenthen J, Jongen JL, Hop WC, Visser GH, et al. (2010) Pain in Guillain-Barre syndrome: a long-term follow-up study. Neurol. 75(16):1439-47.
- Moulin DE, Hagen N, Feasby TE, Amireh R, Hahn A. (1997) Pain in Guillain-Barré syndrome. Neurol. 48(2):328-31.
- Ropper AH, Shahani BT. (1984) Pain in Guillain-Barré syndrome. Arch Neurol. 41(5):511-4.
- Pithadia AB, Kakadia N. (2010) Guillain-Barré syndrome (GBS). PR. 62(2):220-32.
- Cornblath DR, Mellits ED, Griffin JW, McKhann GM, Albers JW, et al. (1988) Motor conduction studies in Guillain-Barré syndrome: description and prognostic value. Ann Neurol. 23(4):354-9.
- Mulkey SB, Glasier CM, El-Nabbout B, Walters WD, Ionita C, et al. (2010) Nerve root enhancement on spinal MRI in pediatric Guillain-Barré syndrome. Pediatr Neurol. 43(4):263-9.
- Storm PB, Chou D, Tamargo RJ. (2002) Lumbar spinal stenosis, cauda equina syndrome, and multiple lumbosacral radiculopathies. Phys Med Reh Clin N. 13(3):713-33.
- Pham HP, Schwartz J. (2019) Therapeutic Plasma Exchange in Guillain-Barre Syndrome and chronic inflammatory demyelinating polyradiculoneuropathy. Presse medicale (Paris, France: 1983). 48(11 Pt 2):338-46.
- Plasmapheresis and acute Guillain-Barré syndrome. The Guillain-Barré syndrome Study Group. Neurol. 35(8):1096-104.
- Raphael JC, Chevret S, Hughes RA, Annane D. (2012) Plasma exchange for Guillain-Barré syndrome. The Cochrane database of systematic reviews. 2012(7):Cd001798.
- Hughes RA, Raphaël JC, Swan AV, Doorn PA. (2004) Intravenous immunoglobulin for Guillain-Barré syndrome. The Cochrane database of systematic reviews. 2004(1):Cd002063.
- Tarlov IM. (1938) Perineurial Cysts of the Spinal Nerve Roots. Arch Neurol Psychiatry. 40(6):1067-74.
- Murphy KP, Ryan S. (2019) Shrinking of a Tarlov cyst. BMJ Case Rep. 12(3):e227256.
- Murphy K, Oaklander AL, Elias G, Kathuria S, Long DM. (2016) Treatment of 213 Patients with Symptomatic Tarlov Cysts by CT-Guided Percutaneous Injection of Fibrin Sealant. Am J Neuroradiol. 37(2):373-9.

