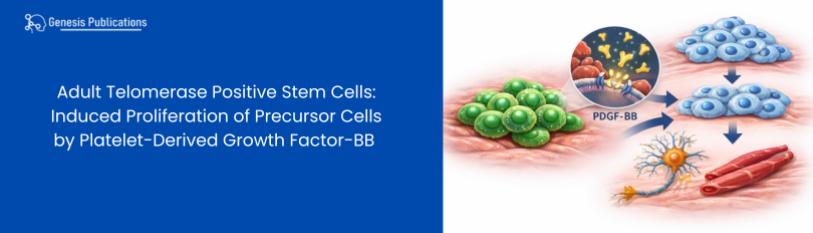
Adult Telomerase Positive Stem Cells: Induced Proliferation of Precursor Cells by Platelet-Derived Growth Factor-BB
Henry E. Young*1-6
1Dragonfly Foundation for Research & Development, Macon, GA 31210 USA
2Henry E Young PHD Regeneration Technologies, Macon, GA 31210 USA
3Division of Basic Medical Sciences, Mercer University School of Medicine, Macon, GA, 31210, USA
4Department of Surgery, Mercer University School of Medicine, Macon, GA, 31210, USA
5Department of Pediatrics, Mercer University School of Medicine, Macon, GA, 31210, USA
6Department of Obstetrics and Gynecology, Mercer University School of Medicine, Macon, GA, 31210, USA
*Corresponding author: Henry E. Young, PhD, Dragonfly Foundation for Research & Development, 101 Preston Ct, Ste 101, Macon, GA 31210 USA, Cell: (478) 319-1983.
Citation: Young HE. Adult Telomerase Positive Stem Cells: Induced Proliferation of Precursor Cells by Platelet-Derived Growth Factor-BB. J Stem Cell Res. 7(1):1-15.
Received: January 10, 2026 Published: January 18, 2026.
Copyright©2026 Genesis Pub by Young HE. This is an open-access article distributed under the terms of the Creative Commons Attribution4.0 International License (CC BY 4.0). This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author(s) and source are properly credited.
DOI: https://doi.org/10.52793/JSCR.2026.7(1)-82
Abstract
Examination of human recombinant proteins, morphogenetic proteins, and cell-specific exosome-conditioned medium with precursor cells noted multiple biological activities, e.g., anti-differentiation, induction, progression, and proliferation. In this study, three single cell-derived clones of rat precursor cells were utilized for testing for proliferation, e.g., pluripotent stem cell (PSC, Scl-40b), mesodermal stem cell (MesoSCs, Scl-A2A2b), and mesenchymal stem cell (MSC, Rt-MSC). Frozen clones were processed for plating into 96-well plates. After 24 hours, plating medium was removed and replaced with testing medium. Testing medium consisted of complete medium without (control) or with (experimental) dose response curves of three biological agents, e.g., 0.5 to 10 mg/ml insulin (Ins), 0.1 to 1000 nM Dexamethasone (Dex), and 0.1 to 500 ng/ml platelet-derived growth factor-BB (PDGF-BB). Sample size for each set of controls was n=144; whereas sample size for each dose response curve was n=18. DNA content of control and experimental groups was measured and evaluated using analysis of variance (ANOVA), with the Newman-Keuls post hoc test for significance.
A p value of less than 0.05 was considered significant. Proliferation was standardized to controls for each clone. Optimum concentration of factors analyzed resulted in 103% in PSCs, 120% in MesoSCs, and 196% in MSCs for insulin; 325% in PSCs, 13% in MesoSCs, and 175% in MSCs for dexamethasone; and 3000% in PSCs, 1978% in MesoSCs, and 1958% in MSCs for PDGF-BB. Therefore, PDGF-BB was deemed the most effective proliferative agent of the three tested using single cell clones of PSCs, MesoSCs, and MSCs as the test subjects.
Keywords
Precursor Cells; Progenitor Cells; Stem Cells; Regenerative Medicine; Platelet-Derived Growth Factor; Proliferation.
Abbreviations
- 2D, two-dimensional
- 3D, three-dimensional
- 370C, thirty-seven degrees centigrade
- a-FGF, acidic-fibroblast growth factor
- aTPSCs, adult telomerase positive stem cells
- Ab/Am, antibiotic (penicillin, streptomycin)/antimycotic (amphotericin-B)
- ACM, adipose conditioned medium
- AMP, adipose morphogenetic protein
- ADF, anti-differentiation factor
- ANOVA, analysis of variance
- b-FGF, basic-fibroblast growth factor
- Bcl-2, acts as an anti-apoptotic regulator
- bME, beta-mercaptoethanol
- BMP-2, bone morphogenetic protein-2
- BMP-4, bone morphogenetic protein-4
- BVCM, blood vessel conditioned medium
- BVMP, blood vessel morphogenetic protein
- BrnCM, brain conditioned medium
- BrnMP, brain morphogenetic protein
- CAF, caffeine
- CardMMP, cardiac muscle morphogenetic protein
- CardMCM, cardiac muscle conditioned medium
- CartMP, cartilage morphogenetic protein
- CartCM, cartilage conditioned medium
- CD10, zinc-dependent metalloprotease inactivates certain growth-simulating peptides, playing roles in cell growth, differentiation, and immune function
- CD13, aminopeptidase, metalloprotease involved in protein metabolism, cell growth, adhesion, and migration
- CD56, neural cell adhesion molecule
- CD66e, carcinoembryonic antigen
- CD90, aka, Thy-1, cell surface glycoprotein, involved in cell-to-cell signaling and adhesion
- CD105, endoglin, receptor for TGF-b, crucial for blood vessel formation
- CD117, c-Kit, cell surface receptor for stem cell factor
- CD123, IL-3 (interleukin-3) receptor
- CD166, activated leukocyte cell adhesion molecule (ALCAM)
- CEA-CAM-1, carcinoembryonic antigen-cell adhesion molecule-1
- c-Kit, a receptor tyrosine kinase
- CLSC, corona-like stem cell
- CNS, central nervous system, brain and spinal cord
- CO2, carbon dioxide
- CxCR4, induces chemotaxis
- Dex, Dexamethasone
- ECGF, endothelial cell growth factor
- ELICA, enzyme-linked immunoculture assay, a high throughput screening assay based on both antibody binding and DNA quantitation
- EGTA, ethylene glycol bis (b-aminoethyl ether)-N, N, N’, N’- tetraacetic acid, a monospecific calcium chelator
- EPO, erythropoietin
- FCM, fibroblast conditioned medium
- FMP, fibroblast morphogenetic protein
- GLSC, germ layer lineage stem cell
- HLSC, halo-like stem cell
- HI, heat inactivated serum
- HGF, hepatocyte growth factor
- IACUC, Institutional Animal Care and Use Committee
- IGF-1, insulin-like growth factor-1
- IGF-2, insulin-like growth factor-2
- INS, insulin
- IL-6, Interleukin-6
- KerCM, keratinocyte conditioned medium
- KerMP, keratinocyte morphogenetic protein
- Lac-Z, gene for insect beta-galactosidase, used as a genomic label to identify cells
- LIF, leukemia inhibitory factor
- LigCM, ligament conditioned medium
- LigMP, ligament morphogenetic protein
- LivCM, liver conditioned medium
- LivMP, liver morphogenetic protein
- LngCM, lung conditioned medium
- LngMP, lung morphogenetic protein
- MesoSC, mesodermal stem cell
- MHC-C1, major histocompatibility complex – class 1, self-recognition molecule
- MSC, mesenchymal stem cell
- mg, micrograms
- mm, microns
- N2, nitrogen
- Nanog, a protein essentially for maintaining pluripotency
- Nanos, a protein essentially for maintaining pluripotency
- NGF, nerve growth factor
- nM, nanomolar
- O2, oxygen
- Oct-3/4, master regulator of pluripotency and self-renewal in stem cells
- OsteoCM, osteogenic conditioned medium
- OsteoMP, osteogenic morphogenetic protein
- PanCM, pancreas conditioned medium
- PanMP, pancreas morphogenetic protein
- PBS, phosphate buffered saline
- PDGF-AA, platelet-derived growth factor-AA
- PDGF-AB, platelet-derived growth factor-AB
- PDGF-BB, platelet-derived growth factor-BB
- PSC, pluripotent stem cell
- RCF, relative centrifugation force
- RGD, tripeptide Arginine (R), glycine (G), aspartic acid (D) sequence found in fibronectin, acting as a key recognition site for cell attachment, migration, and tissue development
- Rt-MSC, mesenchymal stem cell clone derived from rat tissue
- ScFMP, scar fibroblast morphogenetic protein
- SerCellCM, Sertoli cell conditioned medium
- Scl-4b, totipotent stem cell sub-clone genomically-labeled with gene for insect beta-galactosidase
- Scl-9b, totipotent stem cell sub-clone genomically-labeled with gene for insect beta-galactosidase
- Scl-40b, pluripotent stem cell sub-clone genomically-labeled with gene for insect beta-galactosidase
- Scl-44b, totipotent stem cell sub-clone genomically-labeled with gene for insect beta-galactosidase
- Scl-A2A2b, mesodermal stem cell sub-clone genomically-labeled with gene for insect beta-galactosidase
- SIF, Scar inhibitory factor
- SkMCM, skeletal muscle conditioned medium
- SkMMP, skeletal muscle morphogenetic protein
- SmMCM, smooth muscle conditioned medium
- SmMMP, smooth muscle morphogenetic protein
- Sonic Hedgehog, essential signaling molecule for embryonic development, guiding cell growth and body patterning
- SSEA-4, stage-specific embryonic antigen-4
- TenCM, tendon conditioned medium
- TenMP, tendon morphogenetic protein
- TGF-beta, transforming growth factor-beta
- Thy-1, aka CD90, cell surface glycoprotein, involved in cell-to-cell signaling and adhesion
- TSC, totipotent stem cell
- VEGF, vascular endothelial cell growth factor
Introduction
Precursor cells are present in the connective tissue interstitium and granulation tissue of postnatal animals, including humans [1]. Examples of these precursor cells are mesenchymal stem cells (MSCs) [2-11], very-small embryonic-like stem cells (VSELs) [12-17], multilineage-differentiating stress-enduring cells (MUSEs) [18-26], marrow-isolated adult multilineage inducible cells (MIAMIs) [27,28], multipotent adult progenitor cells (MAPCs) [29-33], small mobile stem cells (SMS) [34,35], and adult telomerase positive stem cells (aTPSCs) [36-58]. These cells provide the building blocks that are necessary to maintain the tissues and organs of the body throughout their lifespan. They also provide the cellular components for tissue replacement and repair.
The majority of the precursor cells in the body are telomerase negative progenitor cells. At birth, there are a defined number of telomeres present at the ends of each chromosome, dependent on species. In humans, the finite doubling number is 70, based on Hayflick’s Limit [59], whereas in rodents the finite doubling number is 6-8 [60]. With each cell division a telomere is lost from the ends of each chromosome, and thus giving the cell a defined population doubling number until the telomere count reaches zero, then the cells undergo pre-programmed senescence and death [61]. Examples of telomerase negative progenitor cells are multipotent hematopoietic stem cells and neural stem cells, tripotent mesenchymal stem cells, and unipotent adipoblasts, chondroblasts, and osteoblasts [50].
Within the population of precursor cells in there body there also resides a very rare population of post-natal adult telomerase positive stem cells. These endogenous adult telomerase positive stem cells (aTPSCs) retain the telomerase enzyme throughout the lifespan of the individual, thus giving these precursor cells essentially an unlimited proliferation potential as long as they remain undifferentiated [62]. However, once they begin to differentiate, they lose the telomerase enzyme and assume all the characteristics of progenitor cells, including a defined population doubling limit, dependent on species [49]. There are eight subdivisions of aTPSCs, e.g., totipotent stem cells, halo-like stem cells, corona-like stem cells, pluripotent stem cells, germ layer lineage stem cells, ectodermal stem cells, mesodermal stem cells, and endodermal stem cells [49]. These subdivisions have been extensively characterized from multiple species [52] and shown to have some rather unique characteristics. Totipotent stem cells (TSCs) are ultra-small, 0.1 to 2-mm in size; they propagate as multiple layers in both 2D or 3D cell cultures; they express CEA-CAM-1/CD66e cell surface markers; their expressed genes are telomerase, Bcl-2, Nanog, Nanos, and CxCR4; they can differentiate into any somatic cell in the body; due to their small size, they can traverse the blood brain barrier at the cribriform plate to restore histoarchitecture and function of damaged cells within the CNS (brain and spinal cord); they can traverse the Thebesian system in the myocardium and restore the vasculature, myocardium, and cardiac skeleton in infarcted hearts from the inside out; they can form the nucleus pulposus of the intervertebral, gender-specific gametes, and extra-embryonic tissues. Halo-like stem cells (HLSCs) are slightly larger than TSCs, >2.0 to 4-mm in size; they express CEA-CAM-1/CD66ehigh / SSEA-4/CD10low cell surface markers; they express the telomerase gene; they propagate as multiple layers in both 2D or 3D cell cultures; they can differentiate into any somatic cell in the body. Corona-like stem cells (CLSCs) are slightly larger than HLSCs, >4.0 to <6-mm in size; they express CEA-CAM-1/CD66elow / SSEA-4/CD10high on their cell surfaces; they express the telomerase gene; they propagate as multiple layers in both 2D or 3D cell cultures; they can differentiate into any somatic cell in the body. Pluripotent stem cells (PSCs) are slightly larger than CLSCs, 6 to 8-mm in size; grow as multiple layers in 2D or 3D cell culture; they express SSEA-4/CD10 cell surface markers; their expressed genes are telomerase, Oct-3/4, and Sonic hedgehog; and they can differentiate into any somatic cell of the body. Germ layer lineage stem cells (GLSCs) are slightly larger than PSCs, >8 to <10 mm in size; they express SSEA-4/CD10high/Thy-1/CD90low cell surface markers; they express the telomerase gene; they propagate to contact inhibition and then stop proliferating in 2D cell culture; they can survive contact inhibition as long as they are fed fresh medium; and they can differentiate into any cell type in the body. Ectodermal stem cells (EctoSCs) are slightly larger than GLSCs, 10-12 mm in size; they express Thy-1/CD90, CD56, and MHC-C1 cell surface markers; they express the telomerase gene; they propagate to contact inhibition in 2D cell culture; they can survive contact inhibition as long as they are fed fresh medium; and they can differentiate into any cell type within the ectodermal germ layer lineage; once they begin to differentiated, they lose expression of the telomerase gene and assume all the characteristics of progenitor cells within their ectodermal lineage, including a defined doubling number until senescence and cell death. Mesodermal stem cells (MesoSCs) are a similar size to EctoSCs, 10-12-mm in size; they express Thy-1/CD90, CD13, and MHC-C1 cell surface markers; they express the telomerase gene; they propagate to contact inhibition in 2D cell culture; they can survive contact inhibition as long as they are fed fresh medium; and they can differentiate into any cell type within the mesodermal germ layer lineage; once they begin to differentiated, they lose expression of the telomerase gene and assume all the characteristics of progenitor cells within their mesodermal lineage, including a defined doubling number until senescence and cell death. Endodermal stem cells (EndoSCs) are a similar size to EctoSCs, 10-12-mm in size; they express Thy-1/CD90 and MHC-C1 cell surface markers; they express the telomerase gene; they propagate to contact inhibition in 2D cell culture; they can survive contact inhibition as long as they are fed fresh medium; they can differentiate into any cell type within the endodermal germ layer lineage; once they begin to differentiated, they lose expression of the telomerase gene and assume all the characteristics of progenitor cells within their endodermal lineage, including a defined doubling number until senescence and cell death [38,41,43,48,49,63,64].
The tripotent progenitor mesenchymal stem cells (MSCs) have also been characterized both as a mixed population of cells [1-10] and as single cell clones [1,49,50,56]. MSCs are 15-30 mm in size; they express Thy-1/CD90, CD105, CD117, CD123, CD166, and MHC-C1 cell surface markers; they are absent of telomerase gene expression; they propagate to contact inhibition in 2D cell culture; they can survive contact inhibition as long as they are fed fresh medium; they can differentiate into adipose tissue, cartilage, and bone; they have distinct species-specific doubling numbers until senescence and cell death, human 70 and rat 8 [49].
To determine the activities of individual cells rather than mixtures of cells, subdivisions aTPSCs and MSCs were cloned from single cells using repetitive single cell clonogenic analysis using cell-specific exosome-conditioned medium [56]. The aTPSC clones were further processed by genomically labeling them with the Lac-Z gene for beta-galactosidase using lipofectin as the transfecting agent [38]. The aTPSC and MSC clones were used to test their response to dose response curves of human recombinant proteins, morphogenetic proteins, and organ/cell-specific exosome-conditioned media [55], using a high throughput screening assay, i.e., Enzyme-Linked Immunoculture Assay (ELICA) [53]. Four biological activities were noted collectively for these compounds, e.g., anti-differentiation, proliferation, progression, and induction (Table 1) [55].
The current report is an example of the dose response analyses performed to test proliferation in clones of PSCs (Scl-40b), MesoSC (Scl-A2A2b), and MSC (Rt-MSC) with three representative compounds from the list of biological activities, e.g., progression (insulin), induction (dexamethasone), and proliferation (platelet-derived growth factor-BB) (Table 1).
|
Attributes |
Anti-Differentiation |
Proliferation |
Progression |
Induction |
|
Compounds |
LIF1 |
PDGF-AA |
Insulin |
Dexamethasone |
|
|
ADF2 |
PDGF-AB |
IGF-13 |
BMP-24 |
|
|
CAF5 |
PDGF-BB |
IGF-26 |
BMP-47 |
|
|
SIF8 |
|
|
a-FGF9 |
|
|
|
|
|
ECGF10 |
|
|
|
|
|
VEGF11 |
|
|
|
|
|
TGF-b12 |
|
|
|
|
|
b-FGF13 |
|
|
|
|
|
NGF14 |
|
|
|
|
|
HGF15 |
|
|
|
|
|
Cell-Spec-MP16 |
|
|
|
|
|
Cell-Spec-CM17 |
Table 1: Lif1, Leukemia Inhibitory Factor; ADF2, Anti-Differentiative Factor; IGF-13, Insulin-Like Growth Factor-1; BMP-24, Bone Morphogenetic Protein-2; CAF5, IGF-26, Insulin-Like Growth Factor-2; BMP-47, Bone Morphogenetic Protein-4; SIF8, a-FGF9, acidic-Fibroblast Growth Factor; ECGF10, Endothelial Cell Growth Factor; VEGF11, Vascular Endothelial Cell Growth Factor; TGF-b12, Transforming Growth Factor-beta; b-FGF13, basic-Fibroblast Growth Factor; NGF14, Nerve Growth Factor; HGF15, Hepatocyte Growth Factor; Cell-Spec-MP16, Cell Specific Morphogenetic Proteins (See Abbreviations); Cell-Spec-CM17, Cell Specific exosome Conditioned Media.
Materials And Methods
The use of animals in this study complied with the guidelines of Mercer University’s Institutional Animal Care and Use Committee (IACUC). These guidelines reflect the criteria for humane animal care of the National Research Council as outlined in “Guide for the Care and Use of Laboratory Animals” prepared by the Institute of Laboratory Animal Resources and published by the National Institutes of Health.
Four-hundred-gram adult male outbred Sprague-Dawley rats [Rattus norvegius, Crl:CD(SD)] were euthanized by CO2 asphyxiation. A five-gram skeletal muscle biopsy was removed and placed into sterile phosphate buffered saline, pH 7.4 (PBS, GIBCO, Grand Island, NY) into a sterile 100-mm glass petri dish (Corning, Corning, NY). Using watchmaker’s forceps (Diagger, Vernon Hills, IL). The biopsy specimen was macerated to the consistency of orange marmalade and then placed into a 50-ml polypropylene centrifuge tube (Falcon, Thermo-Fisher, Waltham, MA). An equal volume of a combinatorial enzyme solution was added to the tube. The combinatorial enzyme solution, i.e., 250 units/ml type-1 collagenase (Worthington Biochemical Corp, Lakewood, NJ) and 33.3 units/ml dispase (R&D Laboratories, Minneapolis, MN) in PBS, without calcium or magnesium (GIBCO), containing, 25 mM ethylene glycol bis (b-aminoethyl ether)-N,N,N’,N’- tetraacetic acid (EGTA, Sigma) (25 mM = 0.475 g EGTA/500 ml calcium-/magnesium-free PBS), pH 7.4, was used to separate precursor cells, e.g., aTPSCs and MSCs, from their extracellular matrices [65]. This occurred at 370C for 60-minutes in a shaker water bath. The enzymatic digestion was halted by adding 1% type-1 collagen solution, the contents centrifuged at 2,000 RCF, and the supernatant decanted to bleach. The cell pellet was reconstituted in complete medium, e.g., OptiMEM + GlutaMax (GIBCO), 10% HI serum (Atlas Biologicals, Fort Collins, CO), 1% antibiotic/antimycotic (Sigma, St Louis, MO), pH 7.4. The cells were counted with 0.4% Trypan blue (Kodak, Rochester, NY) in species-specific buffer (pH 7.4), diluted to plating density of 10^6 cells per ml and 2.5-ml plated into 1% collagen-coated T-25 flasks (Falcon). Cells were allowed to attach for 24 hours, the plating medium removed and replaced with propagation medium. Propagation medium consisted of complete medium containing 10 ng/ml PDGF-BB. Cultures were propagated to multi-layered confluence. Medium was replaced when color of culture medium changed from salmon-colored to orange-yellow, which occurred from days to hours, depending on the density of the cells in the flasks [65]. When medium changes occurred approximately every 6-hours, the cells were removed from the flasks. The procedure consisted of separating the attached cells from their calcium-dependent and RGD-(fibronectin)-dependent binding sites. This is accomplished by removing the culture medium, washing the cells 2-3 times with 5-ml of species-specific buffer (PBS), removal of the wash buffer, and incubating the cells in ½ volume wash buffer with calcium/magnesium-free PBS containing 25 mM EGTA. EGTA is a mono-specific calcium chelator, so as the calcium-dependent binding sites are disrupted, the cells begin to round up (takes ~1-5 minutes). This is then followed with removal of the EGTA-buffer and replacement with similar volume of collagenase/dispase enzymatic release solution (containing EGTA). The release solution contains the same concentration of enzymes that was used to release the cells from the macerated tissue. Trituration was used to derive single cell suspensions as cells were released from flask surface (as individual cells or as sheets of cells) (takes ~ 30-60 seconds). The cell suspension is placed into a fresh 50-ml polypropylene centrifuge tube and 1% type-1 collagen solution added at a v/v ratio of 1:14, cell suspension to collagen solution. The solution was triturated 3-4 times and the suspended cells are spun at 2,000 RCF to pellet the cells. The supernatant is removed to bleach and the cells resuspended in PBS, pH 7.4 [65].
Cell sorting using cell-specific cell surface markers, e.g., Thy-1 for MSCs and GLSCs, EctoSCs, MesoSCs, and EndoSCs; SSEA-4 for HLSCs, CLSCs, and PSCs; and CEA-CAM-1 for TSCs, HLSCs, and CLSCS was used as described [66]. Three negative sorts were used for each group. For TSCs, 1st sort was with Thy-1 to remove MSCs, GLSCs, EctoSCs, MesoSCs, and EndoSCs; second sort was with SSEA-4 to remove PSCs, CLSCs, and HLSCs; and third sort used CEA-CAM-1 to purify TSCs. For PSCs, 1st sort was with Thy-1 to remove MSCs, GLSCs, EctoSCs, MesoSCs, and EndoSCs; 2nd sort was with CEA-CAM-1 to remove TSCs, HLSCs, and CLSCs; and third sort used SSEA-4 to purify PSCs. For MSCs, GLSCs, EctoSCs, MesoSCs, and EndoSCs, the first sort was with SSEA-4 to remove PSCs, CLSCs, and HLSCs; second sort used CEA-CAM-1 to separate TSCs, HLSCs, and CLSCs; and third sort used Thy-1 to purify MSCs, GLSCs, EctoSCs, MesoSCs, and EndoSCs.
Cells were then processed for repetitive serial dilution clonogenic analysis as described [56]. Multiple aTPSC and MSC clones were generated and subsequently characterized for previously defined attributes, e.g., size, Trypan blue staining, cell surface markers, differentiation characteristics, etc. [49]. The aTPSC clones were further processed by genomic labeling using the Lac-Z gene for insect beta-galactosidase with lipofectin as the transfection agent [56]. The aTPSC clones were genomically-labeled in such a manner, that as undifferentiated cells the reaction product of the genomic label was located in the nucleus. With differentiation of the cell, the reaction product of the genomic label translocated to the cytoplasm of the cell [38,48]. Aliquots of unlabeled and genomically-labeled clones were cryopreserved [66] for testing. We characterized five of 58 genomically-labeled clones thus far, TSC clones Scl-4b, Scl-9b, and Scl-44b; PSC clone Scl-40b; and MesoSC clone Scl-A2A2b. Frozen aliquots of the PSC clone Scl-40b; the MesoSC clone Scl-A2A2b; and the MSC clone Rt-MSC were used for this study. Frozen aliquots were thawed at 370C in a controlled temperature water bath. When the color of the medium changed from bright yellow (frozen) to salmon-color (thawed), 1.0-ml cell suspension was removed from the cryovials were and placed into 15-ml polypropylene centrifuge tubes (Falcon). Complete medium was added at a v/v ratio of 14:1 medium to cell suspension, and contents centrifuged at 1,500 RCF for PSC clone, 1,000 RCF for MesoSC clone, and 500 RCF for MSC clone, respectively, to remove DMSO from the cells [66]. The cells were reconstituted in complete medium, counted, diluted to appropriate density in complete medium and then plated at 10^3 cells per well in 1% type-1 collagen-coated (EM Sciences, Gibbstown, NJ) 96-well plates (Costar, Corning, Corning, NY), and placed into a humidified 370C 5% CO2/95% air (ambient O2, balanced N2) incubator for 24 hours. Plating in complete medium for the three clones consisted of 89% (v/v) Opti-MEM + GlutaMax (GIBCO, Grand Island, NY), 1% (v/v) antibiotic-antimycotic solution (10,000 units/ml penicillin; 10,000 micro grams per ml streptomycin; 25 micro grams amphotericin-B, GIBCO) (1% Ab/Am), 10% Heat Inactivated Serum (HI Serum, Atlas Biologicals, Fort Collins, CO), at pH 7.4. Non-heat inactivated serum contains inductive, progressive, proliferative, and/or anti-differentiation agents that will alter the results of any experiment [65]. To deactivate the potential effects of these biological agents, the serum was heat treated. This occurred at 56-600C for 4-8 hours, followed by high-speed centrifugation (100,000+ RCF), followed by positive filtration for sterilization though a 0.1-micron steel mesh filter (Atlas Biologicals).
Twenty-four hours after attachment of the cells, the plating medium was removed and replaced with optimal testing medium for each cell type. The aTPSCs, PSCs (and TSCs) prefer an anerobic (hypoxic) environment for stasis and proliferation. Therefore, for the PSC clone Scl-40b, 0.01-mM b-mercaptoethanol (bME, Sigma, St Louis, MO) was added to the complete medium formulation to reduce oxygen tension and thereby simulate an anerobic environment. [If human cells are used instead and destined for human transplants, then bME is not added to the medium. Rather, the human cells are grown inside a heated, humidified sterile environment, such as a BioSpherix system, where the gas ratios can be consistently maintained at 5% CO2, 5% O2, and 90% N2.] In contrast, MesoSCs and MSCs prefer an aerobic environment for stasis, proliferation, induction, and progression, so bME is NOT added to the testing medium to lower the oxygen saturation. [If human cells are used instead and destined for human transplants, the human cells are grown inside a heated, humidified sterile environment, such as a BioSpherix system, where the gas ratios can be consistently maintained at 5% CO2, 21% O2, and 74% N2.].
Three clones, e.g., PSC clone Scl-40b, MesoSC clone Scl-A2A2b, and MSC clone Rt-MSC, were tested with three compounds using a dose response curve analysis, n=18 repetitions for each dose response curve. The dose response curves for each of the three compounds were 0.5 to 10 mg/ml insulin (0.5, 1.0, 2.0, 5.0, and 10.0) (Sigma); 10-6 to 10-10 M Dexamethasone (0.1, 1.0, 10, 100, and 1,000 nM) (Sigma); and 0.1 to 500 ng/ml platelet-derived growth factor-BB (0.1, 0.2, 0.5, 1.0, 2, 5, 10, 20, 50, 100, 200, and 500 ng/ml PDGF-BB) (R&D Systems, Minneapolis, MN).
DNA Analysis
Cultures were treated for an additional eight days with plating medium containing the dose response curves. One half of the medium was replaced every three days. Control cultures (8 x experimental numbers, n=18 x 8 = 144) were run for all experimental testing. In control cultures, the testing medium was void of any additional agents. The cultures were assayed for DNA content as a measure of cellular proliferation by means of a fluorescent procedure utilizing diaminobenzoic acid [53,67]. Experiments were run with a sample size of 18 for each concentration for each factor examined. DNA content after treatment was evaluated using analysis of variance (ANOVA), using the Newman-Keuls post hoc test for significance. A p value of less than 0.05 was considered significant. The statistical analyses were performed using the ABSTAT computer program (Anderson Bell Corp., Arvada, CO).
Results
Control media void of biological activities, from each dose response curve tested on the three precursor cells was averaged (n=144) and used to establish baseline values of cellular proliferation by measuring micrograms of DNA content per well (Tables 2-4). Statistically significant values from controls had p values of <0.05. Incubation with 0.5 to 10 micrograms of insulin produced no statistically significant increase in DNA content in PSCs at 103% of control, (Table 2) or MesoSCs at 120% of control (Table 3). However, 0.2 micrograms per ml insulin did produce a significant increase in DNA content in MSCs at 196% of control (Table 4).
Dexamethasone produced a statistically significant increase in DNA content in PSCs only at a concentration of 0.1nM, with 325% of control value (Table 2); it produced a reduction in DNA content in cultures MesoSCs at 0.1nM Dex, i.e., 13% (Table 3); while incubation of MSCs with 0.1nM Dex caused a significant increase in DNA content at 175% of control (Table 4).
PDGF-BB produced a statistically significant increase in DNA content in all three precursor cells tested, e.g., 3000% in PSCs (Table 2), 1978% in MesoSCs (Table 3), and 1958% in MSCs (Table 4). Based on dose response curves for the biological agents tested, different optimum concentrations for proliferation, i.e., average increase in DNA content per well, were noted dependent on the cells examined. For example, optimal concentration for insulin was at a concentration of 2.0 mg/ml for MesoSCs and MSCs, but 10 mg/ml for PSCs; optimum concentration of Dex for PSCs, MesoSCs, and MSCs was 0.1nM; whereas optimum concentration of PDGF-BB was 200 ng/ml for PSCs and 100 ng/ml for both MesoSCs and MSCs (Table 5).
|
Bioactive Factor |
Concentration |
mg DNA + SD |
% of Control |
Sample Size |
|
Control |
0.0 ng/ml |
0.035 + 0.053 |
100 |
144 |
|
Insulin |
10.0 mg |
0.036 + 0.056 |
103 |
18 |
|
Dexamethasone |
0.1nM |
0.114 + 0.065* |
325 |
18 |
|
PDGF-BB |
200 ng/ml |
1.050 + 0.112* |
3000 |
18 |
*Statistically significant from control, p value < 0.05.
Table 2: Maximal Proliferation Response of Pluripotent Stem Cell Clone (Scl-40b) to Insulin, Dexamethasone, and Platelet-Derived Growth Factor-BB.
|
Bioactive Factor |
Concentration |
mg DNA + SD |
% of Control |
Sample Size |
|
Control |
0.0 ng/ml |
0.500 + 0.224 |
100 |
144 |
|
Insulin |
2.0 mg |
0.599 + 0.171 |
120 |
18 |
|
Dexamethasone |
0.1nM |
0.064 + 0.109 |
13 |
18 |
|
PDGF-BB |
100 ng/ml |
9.888 + 0.318* |
1978 |
18 |
*Statistically significant from control, p value < 0.05.
Table 3: Maximal Proliferation Response of Mesodermal Stem Cell Clone (Scl-A2A2b) to Insulin, Dexamethasone, and Platelet-Derived Growth Factor-BB.
|
Bioactive Factor |
Concentration |
mg DNA + SD |
% of Control |
Sample Size |
|
Control |
0.0 ng/ml |
0.500 + 0.075 |
100 |
144 |
|
Insulin |
2.0 mg |
0.980 + 0.137* |
196 |
18 |
|
Dexamethasone |
0.1nM |
0.876 + 0.152* |
175 |
18 |
|
PDGF-BB |
100 ng/ml |
9.788 + 0.231* |
1958 |
18 |
*Statistically significant from control, p value < 0.05.
Table 4: Maximal Proliferation Response of Mesenchymal Stem Cell Clone (Rt-MSC) to Insulin, Dexamethasone, and Platelet-Derived Growth Factor-BB.
|
Bioactive Factor |
Maximum Concentration |
PSC (Scl-40b) |
MesoSC (Scl-A2A2b) |
MSC (Rt-MSC) |
|
Control |
0.0 ng/ml |
100 |
100 |
100 |
|
Insulin |
2.0 mg |
103 |
120 |
18 |
|
Dexamethasone |
0.1nM |
325 |
13 |
18 |
|
PDGF-BB |
100 ng/ml |
3000 |
1978 |
18 |
*Statistically significant from control, p value < 0.05.
Table 5: Maximal Proliferation Responses of Clones of Pluripotent Stem Cells, Mesodermal Stem Cells, and Mesenchymal Stem Cells to Insulin, Dexamethasone, and Platelet-Derived Growth Factor-BB.
Discussion & Conclusion
Previous studies have addressed the influence of various biological factors on endogenous adult precursor cells, e.g., telomerase positive stem cells and telomerase negative progenitor cells [1]. There are eight categories of aTPSCs, based on size, Trypan blue staining, cell surface markers, and differentiation activities [49]. The eight categories are TSCs, HLSCs, CLSCs, PSCs, GLSCs, EctoSCs, MesoSCs, and EndoSCs. MSCs were also isolated concurrent with the aTPSCs. These cells were derived as initial isolates from solid tissue biopsy specimens using collagenase and dispase to release precursor cells from their respective extracellular matrices [65]. Clones of both aTPSCs and MSCs were generated by repetitive single cell clonogenic analysis [66]. The aTPSCs were further genomically-labeled to recognize the respective differentiated cell types from non-genomically-labeled progenitor cells [38]. The biological agents examined were two chemicals, 18 human recombinant proteins, 19 morphogenetic proteins, and 16 cell/organ-specific exosome conditioned media [55,68]. Based on the results from these studies, four biological activities were noted by visual inspection, e.g., anti-differentiative (LIF, ADF, CAF, SIF), proliferative (PDGF-AA, PDGF-AB, PDGF-BB), progressive (insulin, IGF-1, IGF-2), and inductive (dexamethasone, BMP-2, BMP-4, a-FGF, ECGF, VEGF, TGF-beta, b-FGF, EPO, IL-6, c-Kit, NGF, HGF, SkMMP, SmMMP, CardMMP, CartMP, OsteoMP, TenMP, LigMP, AMP, FMP, ScFMP, BVMP, BrnMP, LivMP, LngMP, PanMP, KerMP, SkMCM, SmMCM, CardMCM, CartCM, OsteoCM, TenCM, LigCM, ACM, FCM, BVCM, BrnCM, LivCM, LngCM, PanCM, KerCM, SerCellCM). To verify the above visual results using objective assays, various combinations of biological agents for anti-differentiation, proliferation, progression, and induction were tested with single cell clones of aTPSCs and MSCs quantifying both phenotypic expression markers of differentiated cell types and DNA content. In this study, the PSC clone Scl-40b, MesoSC clone Scl-A2A2b, and MSC clone Rt-MSC were incubated with dose response curves of insulin, dexamethasone, and PDGF-BB. As shown in Tables 2-5, PDGF-BB was the most potent stimulator of proliferation, with DNA contents of 3000% (PSC Scl-40b), 1978% (MesoSC Scl-A2A2b) and 1958% (Rt-MSC) of their respective control values. Of interest is the difference in DNA content between PSCs that will form multiple confluent layers of cells in culture versus MesoSCs and MSCs that stop proliferating once single layer confluence is reached. This difference in DNA content is reflected by the measured quantities of DNA from the respective cells.
Acknowledgements
The author would like to thank JA F-C-Coleman, GF L-Black, NL Henson, and S Jackson for cell culture; JA F-C-Coleman, GF L-Black, and NL Henson for ELICA analyses; M Rimando, J-I Yoon, and Dr. C Bridges for technical assistance; Dr RK Zalups for use of his Victor Fluorometer (Wallac, PerkinElmer, Waltham, MA); and S Jackson and Dr AC Black Jr for statistical analyses.
Funding
Funding for this work was supported by research grants from Rubye Ryle Smith Charitable Trust, The MEDCEN Community Health Foundation of Central Georgia, Dragonfly Foundation for Research and Development, and Morphogen Pharmaceuticals, Inc.
Competing and conflicting interests
Endogenous adult telomerase positive stem cells (aTPSCs) have competing interests with those laboratories studying mixed populations of telomerase negative progenitor cells termed stem cells, e.g., mesenchymal stem cells (MSCs), very small-embryonic-like stem cells (VSELs), multilineage-differentiating stress-enduring cells (MUSEs), marrow-isolated adult multilineage inducible cells (MIAMIs), small mobile stem cells (SMSs), and multipotent adult progenitor cells (MAPCs). The conflicting interest relates to comparing innate cellular characteristics, putative differentiation potentials and ability to affect a positive regenerative response when transplanted in vivo of single cell-derived clones of aTPSCs versus mixed cell populations of MSCs, VSELs, MUSEs, MIAMIs, SMSs, and MAPCs.
Nomenclature
Sprague-Dawley rat, Rattus norvegius, Crl:CD(SD)
References
- Young HE. (2025) Adult Telomerase Positive Stem Cells: Compare and Contrast Biobanking with Mesenchymal Stem Cells and Other Progenitor Cells. GSC Adv ResRev. 25(03):218-26.
- Friedenstein AJ, Deriglasova UF, Kulagina NN. (1974) Precursors for fibroblasts in different populations of hematopoietic cells as detected by the in vitro colony assay method. Exp Hematol. 2:83-92.
- Da Silva ML, Chagastelles PC, Nardi NB. (2006 Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 119:2204-213.
- Caplan AI. (1991) Mesenchymal stem cells. J Orthop Res. 9:641-650.
- Caplan AI. (2005) Review: Mesenchymal stem cells: cell-based reconstructive therapy in orthopedics. Tissue Eng. 11:1198-1211.
- Pittenger MF, Mackay AM, Beck SC. (1999) Multilineages potential of adult human mesenchymal stem cells. Science. 284:143-47.
- Caplan AI. (2017) Mesenchymal stem cells: time to change the name. Stem Cells Translational Medicine. 6(6): 1445-1451.
- Caplan AI, Dennis JE. (2006) Mesenchymal stem cells as trophic mediators. J Cell Biochemistry. 98(5): 1076-1084.
- Li Z, Hu X, Zhong JF. (2019) Mesenchymal stem cells: characteristics, function, and application. Stem Cells Intl. 6:8106818.
- Via AG, Frizziero A, Oliva F. (2012) Biological properties of mesenchymal stem cells from different sources. Muscles Ligaments Tendons J. 2(3):154-162.
- Young HE. (2025) Adult telomerase positive stem cells: differential cryopreservation, cell surface marker profiles, and cell sorting. MOJ Orthopedics & Regeneration. 17(5):155-73.
- Ratajczak MZ, Ratajczak J, Kucia M. (2019) Very small embryonic-like stem cells (VSELs) – an update and future directions. Circ Res. 124(2):208-210.
- Zuba-Suma EK, Wojakowski W, Ratajczak MZ, Dawn B. (2011) Very small embryonic-like stem cells: biology and therapeutic potential for heart repair. Antioxid Redox Signal. 15(7):1821-34.
- Ratajczak MZ, Zuba-Suma E, Wojakowski W, Kucia M, et al. (2014) Very small embryonic-like stem cells (VSELs) represent a real change in stem cell biology: recent pros and cons in the midst of a lively debate. Leukemia. 28: 473-484.
- Danova-Alt R, Heider A, Egger D, Cross M, Alt R. (2012) Very small embryonic-like stem cells purified from umbilical cord blood lack stem cell characteristics. PLoS ONE. 7(4): e34899.
- Ratajczak MZ, Shin D-M, Lui R, Mierzejewska K, Ratajczak J, et al. (2012) Very small embryonic/epiblast-like stem cells (VSELs) and their potential role in aging and organ rejuvenation – an update and comparison to other primitive small stem cells isolated from adult tissues. Aging. 4(4):235-46.
- Thetchinamoorthy K, Jarczak J, Kieszek P, Wierzbicka D, Ratajczak J, et al. (2025) Very small embryonic-like stem cells (VSELs) on the way for potential applications in regenerative medicine. Front Bioeng Biotechnol. 13:1564964.
- Wakao S, Kitada M, Kuroda Y, Dezawa M, et al. (2011) Multilineage-differentiating stress enduring (Muse) cells are a primary source of induced pluripotent stem cells in human fibroblasts. Proc Natl Acad Sci USA. 108(24):9875-9880.
- Ossanna R, Veronese S, Sierra LAQ, Conti A, Conti G, et al. (2023) Multilineage-differentiating stress enduring (Muse) cells: an easily accessible, pluripotent stem cell niche and powerful properties for regenerative medicine applications. Biomedicines. 11(6):1587.
- Que H, Mai E, Hu Y, Li H, Zheng W, et al. (2024) Multilineage-differentiating stress enduring (Muse) cells: a powerful tool for tissue repair. Front Cell Dev Biol. 12:1380785.
- Dezawa M. (2018) The Muse cell discovery, thanks to wine and science. Adv Exp Med Biol. 1103;1-11.
- Alanazi RF, Alhwity BS, Almahlawi RM, Alatawi BD, Albalawi SA, et al. (2023) Multilineage-differentiating stress enduring (Muse) cells: a new era of stem cell-based therapy. Cells. 12(13):1676.
- Yamamoto S, Shiraishi K, Kushida Y, Oguma Y, Wakao S, et al. (2025) Nose-to-brain delivery of human muse cells enhances structural and functional recovery in the murine ischemic stroke model. Sci Rep. 15:16243.
- Velasco MG, Satue K, Chicharro D, Martins E, Rubio M, et al. (2023) Multilineage-differentiating stress enduring (Muse) cells: the future of human and veterinary medicine. Biomedicines. 11(2):636.
- Minatoguchi S, Fujita Y, Niizuma K, Tominaga T, Yamashita T, et al. (2024) Donor muse cell treatment without HLA-matching tests and immunosuppressant treatment. Stem Cells Transl Med. 13(6):527-45.
- Dezawa M. (2025) Macrophage- and pluripotent-like reparative muse cells are unique endogenous stem cells distinct from other somatic stem cells. Front Bioeng Biotechnol. 13:1553382.
- Grau-Monge C, Delcroix GJ-R, Bonnin-Marwuez A, Valdes M, D’lppolito GD, et al. (2017) MIAMI cells embedded within a biologically-inspired construct promote recovery in mouse model of peripheral vascular disease. Biome Mater. 12(1):015024.
- D’lppolito G, Diabira S, Howard GA, Menei P, Roos BA, et al. (2004) Marrow isolated adult multilineage inducible (MIAMI) cells, a unique population of postnatal young and old human cells with extensive expansion and differentiation potential. J Cell Sci. 117(Pt 14): 2971-81.
- Williams R. (2007) Cell replacement therapy – are MAPCs the answer? J Exp Med. 204(1):4.
- Subramanian K, Geraerts M, Pauwelyn KA, Park D, Owens J, et al. (2010) Isolation procedures and characterization of multipotent adult progenitor cells from rat bone marrow. Cell Program Reprogram Meth Prot. 55-78.
- Reyes M, Verfaillie CM. (2001) Characterization of multipotent adult progenitor cells, a subpopulation of mesenchymal progenitor cells. Ann NY Acad Sci. 938:231-233.
- Verfaillie CM. (2005) Multipotent adult progenitor cells: an update. Hematology Am Soc Hematol Edu Program. 265:55-65.
- Jiang Y, Breyer A, Lien L, Blackstad M, Verfaillie C. (2004) Culture of multipotent adult progenitor cells (MAPCs). Blood. 104(11): 2329.
- Smsbiotech.com
- Rahmo A, Schechter R. (2024) Small Mobile Stem Cells, Unique Regenerative Effects on Lung of Emphysema Animal Model Currently Undergoing Phase 1 Clinical Trial. Am J Respir Crit Care Med. 209: A6431.
- Young HE, Steele T, Bray RA, Detmer K, Blake LW, (1999) Lucas PA, Black AC Jr. Human progenitor and pluripotent cells display cell surface cluster differentiation markers CD10, CD13, CD56, CD90 and MHC Class-I. Proc Soc Exp Biol Med. 221:63-71.
- Young HE, Black Jr AC. (2004) Adult stem cells. Anat Rec. 276A:75-102.
- Young HE, Duplaa C, Romero-Ramos M, Chesselet MF, Black Jr AC, et al. (2004) Adult reserve stem cells and their potential for tissue engineering. Cell Biochem Biophys. 40(1):1-80.
- Young HE, Duplaa C, Yost MJ, Black Jr AC. (2004) Clonogenic analysis reveals reserve stem cells in postnatal mammals. II. Pluripotent epiblastic-like stem cells. Anat Rec. 277A:178-203.
- Henson NL, Heaton ML, Holland BH, Young HE, et al. (2005) Karyotypic analysis of adult pluripotent stem cells. Histology and Histopathology. 20:769-84.
- Young HE, Duplaa C, Katz R, Thompson T, Black AC Jr, et al. (2005) Adult-derived stem cells and their potential for tissue repair and molecular medicine. J Cell Molec Med. 9:753-769.
- Stout CL, Ashley DW, Morgan III JH, Young HE, et al. (2007) Primitive stem cells reside in adult swine skeletal muscle and are mobilized into the peripheral blood following trauma. American Surgeon. 73 (11):1106-1110.
- Young HE, Hyer L, Black AC Jr, Robinson Jr JS. (2013) Treating Parkinson disease with adult stem cells. J Neurol Disorders. 2:107-109.
- Young HE, Limnios JI, Lochner F, McCommon G, Cope LA, et al. (2017) Pancreatic islet composites secrete insulin in response to a glucose challenge. J Stem Cell Res. 1(1) 001: 1-12.
- Young HE, Lochner F, Lochner D, Lochner D, McCommon G, et al. (2017) Primitive Stem Cells in Adult Feline, Canine, Ovine, Caprine, Bovine, and Equine Peripheral Blood. J Stem Cell Res. 1(1) 004:1-6.
- Young HE, Lochner F, Lochner D, Lochner D, Black GF, et al. (2017) Primitive stem cells in adult human peripheral blood. J Stem Cell Res. 1(2) 001:1-8.
- Young HE, Black GF, Coleman JA, Hawkins KC, Black Jr AC. (2017) pulmonary diseases and adult healing cells: from bench top to bedside. J Stem Cell Res. 1(2) 003:1-9.
- Young HE, Limnios IJ, Lochner F, McCommon G, Black GF, et al. (2017) cardiovascular disease and adult healing cells: From bench top to bedside. J Stem Cell Res. 1(3) 002:1-8.
- Young HE, Speight MO. (2020) Characterization of endogenous telomerase-positive stem cells for regenerative medicine, a review. Stem Cell Regen Med. 4(2):1-14.
- Young HE. (2023) Fresh Isolate Adult Telomerase Positive Stem Cells: An addition to Embryonic Stem Cells (ESCs), Induced Pluripotent Stem Cells (iPSCs), and/or Mesenchymal Stem Cells (MSCs) for Regenerative Medicine. GSC Adv Res Rev. 16(1):066-081.
- Young HE. (2024) Combinatorial Nutraceutical Supplement Pill (CNSP) Stimulates Naïve Adult Telomerase Positive Stem Cells In-Situ to Heal Cardiomyopathies. GSC Adv Res Rev. 20(02), 047-056.
- Young HE. (2025) Totipotent stem cells and pluripotent stem cells are present in the reproductive organs of an adult mammal. GSC Adv Res Rev. 23(03): 158-180.
- Young HE. (2025) A high throughput screening assay to quantify, visualize, and standardize biological activities: Enzyme-Linked Immuno-Culture Assay (ELICA). GSC Adv Res Rev. 24(02):091-114.
- Young HE. (2025) Adult telomerase positive stem cells: introduction and location. GSC Adv Res Rev. 25(02): 296-331.
- Young HE. (2025) Adult telomerase positive stem cells: Effects of Biological Agents on Single Cell Clones of Adult Cells. GSC Adv Res Rev. 25(03): 028-047.
- Young HE. (2025) Adult telomerase positive stem cells: Cell-Specific Exosome-Conditioned Medium, Repetitive Single Cell Clonogenic Analysis, and Genomic Labeling. GSC Adv Res Rev. 25(2): 480-504.
- Young HE. (2025) Adult telomerase positive stem cells: differential cryopreservation, cell surface marker profiles, and cell sorting. MOJ Orthopedics & Regeneration. 17(5): 155-73.
- Rogers JJ, Adkison LR, Black AC Jr, Lucas PA, Young HE. (1995) Differentiation factors induce expression of muscle, fat, cartilage, and bone in a clone of mouse pluripotent mesenchymal stem cells. Amer Surgeon. 61(3):231-236.
- Hayflick L. (1965) The limited in vitro lifetime of human diploid cell strains. Exp Cell Res. 37:614-36.
- Rohme D. (1981) Evidence for a relationship between longevity of mammalian species and life spans of normal fibroblasts in vitro and erythrocytes in vivo. Proc Natl Acad Sci USA. 78:5009-13.
- Zvereva MI, Shcherbakova DM, Dontsova OA. (2010) Telomerase: structure, functions, and activity regulation. Biochemistry (Moscow). 75(13):1563-83.
- Young HE. (2025) Adult Telomerase Positive Stem Cells: Remain Constant Throughout Lifespan of Individual. GSC Advanced Research and Reviews. 25(2):480-504.
- Young HE, Speight MO. (2021) Treating Parkinson Disease with Autologous Telomerase-Positive Stem Cells, Update 2021. Stem Cells Regen Med. 5(1):1-13.
- Young HE, Speight MO. (2020) cardiovascular disease treated with telomerase-positive stem cells. Stem Cells Regen Med. 4(2):1-8. 20-051.
- Young HE. (2025) Adult telomerase positive stem cells: isolation, plating, and propagation. GSC Advanced Research and Reviews. 25(02):407-40.
- Young HE. (2025) Adult telomerase positive stem cells: differential cryopreservation, cell surface marker profiles, and cell sorting. MOJ Orthop Regen. 17(5):155-73.
- Young HE, Sippel J, Putnam LS, Lucas PA, Morrison DC. (1992) Enzyme-linked immuno-culture assay. J Tiss Cult Meth. 14:31-36.
- Young HE. (2025) Adult Telomerase Positive Stem Cells: Effects of Biological Agents on Genomically-Labeled Clones and Unlabeled Clones of Adult Cells. GSC Adv Res Rev. 25(03):127-45.

