The Scummy Canker Mucinous Carcinoma Ovary
Anubha Bajaj*
Department of Histopathology, AB Diagnostics, New Delhi, India
*Corresponding author: Bajaj A, Department of Histopathology, AB Diagnostics, New Delhi, India.
Citation: Bajaj A. (2022) The Scummy Canker Mucinous Carcinoma Ovary. Genesis J Surg Med. 1(1):1-04.
Received: September 09, 2022 | Published: September 30, 2022
Copyright© 2022 Genesis Pub by Bajaj A. This is an open-access article distributed under the terms of the Creative Commons Attribution4.0 International License (CC BY 4.0). This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author(s) and source are properly credited.
DOI: https://doi.org/10.52793/GJSM.2022.1(1)-4
Editorial
Mucinous neoplasms are epithelial ovarian tumours designated as such on account of tumefaction pervaded with mucus-like substance secreted by mucus-secreting goblet cells. The neoplasm can enlarge significantly and is categorized into benign, borderline and malignant lesions. Mucinous tumours constitute ~one third of ovarian surface epithelial-stromal neoplasms (1,2). Factors inducing mucinous ovarian carcinoma appear as enhanced ovulation, nulliparous state, early menarche’, delayed menopause, hormonal replacement therapy, obesity, age >70 years, elevated levels of c-reactive protein (CRP) or ingestion of infertility medications (1,2).Smoking contributes significantly to emergence of mucinous tumours. An inherited genetic predilection is observed with mutations of BRCA1 and BRCA2 genes (1,2). Ovarian carcinoma can occur in first-degree relatives of females with ovarian cancer. Hereditary nonpolyposis colorectal cancer or Lynch syndrome is associated with enhanced possible emergence of ovarian carcinoma (1,2). Oral contraceptives, tubal ligation and breast feeding decimates occurrence of mucinous tumours (1,2).Genetic concurrence of ovarian carcinoma occurs with ESR2, BRIP1, MSH6, RAD50, RAD51C, RAD51D, CDH1, CHEK2 OR PALB2 genes (1,2). Mucinous adenocarcinoma or mucinous cystadeno carcinoma can be painless or devoid of preliminary, disease-specific symptoms and the condition may be misinterpreted as irritable bowel syndrome (1,2). Clinical symptoms are contingent to tumour subtype (1,2). Borderline mucinous neoplasms of low malignant potentialtypically represent with abdominal distension or pelvic pain (1,2). Characteristically, mucinous ovarian carcinomas display abdominal distension, abdomino-pelvic or lumbar pain, discomfort, irregular menstruation, postmenopausal vaginal bleeding, dyspareunia, anorexia, nausea, fatigue, indigestion, heartburn, constipation, diarrhoea, bloating or urinary symptoms as polyuria with urgent micturition (1,2). Enlarging ovarian tumefaction secondary to ovarian torsion can be painful. Also, abdominal mass compressing adjacent abdominopelvic organs or accompanying distant metastasis appear symptomatic (1,2).
The multilocular, benign mucinous tumour exhibits cysts layered by smooth, mucin-secreting epithelium simulating endocervical or gastrointestinal epithelium (1,2). Border line and malignant mucinous neoplasms depict papillary configurations, solid tissue articulations, focal haemorrhage and necrosis (1,2). Malignant mucinous tumours comprised of mucinous adenocarcinoma and mucinous cystadeno carcinoma enunciate a solid configuration with cellular and nuclear atypia, epithelial stratification and pseudo-stratification, focal necrosis and architectural disarray (1,2). Mucinous adenocarcinoma is morphologically identical to intestinal or cervical adenocarcinoma and may represent as metastases of appendiceal or colonic carcinoma (1,2). Specifically, stromal invasion segregates borderline lesions from malignant tumefaction. Malignant metamorphosis may be focal. Exceptionally, tumefaction is bilateral. Bilateral mucinous carcinoma may indicate ovarian metastases of a distant, primary mucinous neoplasm (1,2). Ovarian carcinoma is categorized as
- Grade I neoplasm which is well differentiated with superior prognosis. Tumour cells simulate normal ovarian epithelial cells and solid foci are absent (1,2).
- Grade II neoplasm is moderately differentiated and comprised of minimally aberrant epithelial tumour cells. Solid foci configure ~50% of neoplasm (1,2).
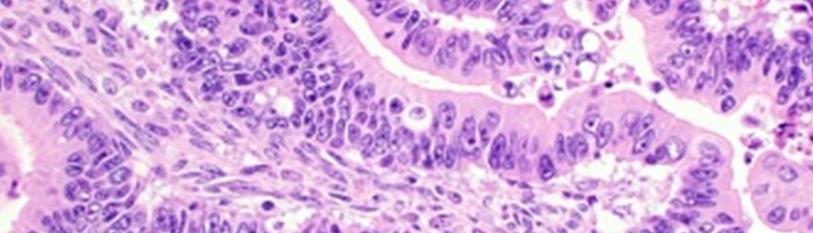
- Grade III tumefaction is poorly differentiated and exemplifies an inferior prognosis. Tumour cells depict cellular and nuclear atypia with stratification, papillary configurations, decimated, complex glandular architecture, solid areas>50% and focal necrosis (1,2). Exceptionally, pseudomyxomaperitonei may occur secondary to an ovarian mucinous neoplasm and appears as an abdomino-pelvic amalgamation of encapsulated, mucinous or gelatinous material (1,2) (Figure 1,2).
Figure 1: Mucinous adenocarcinoma demonstrating epithelial glands lined by atypical, hyperchromaticepithelium with nuclear hyperplasia and few mitotic figures.
Figure 2: Mucinous carcinoma exhibiting atypical glands with partly solid areas and infiltration into surrounding stroma.
International Federation of Obstetrics and Gynaecology (FIGO) stages mucinous carcinoma ovary as Primary Tumour.
TX: Primary tumour cannot be assessed.
T0: No evidence of primary tumour.
T1a (IA): Tumefaction confined to singular ovary with intact capsule or fallopian tube, tumour upon ovarian or fallopian tube surface is absent, ascites or peritoneal washings lack malignant cells.
T1b (IB): Tumour within bilateral ovaries with intact capsule or fallopian tubes, tumour upon ovarian or fallopian tube surface is absent, ascitesor peritoneal washings lack malignant cells.
T1c (IC): Tumour confined to singular or bilateral ovaries or fallopian tubes along with ~T1c1; (IC1): surgical spill of malignant cells~T1c2; (IC2): capsule rupture prior to surgery or tumour superimposed upon ovarian or fallopian tube surface~T1c3;; (IC3): malignant cells discernible in ascites or peritoneal washings.
T2a (IIA): Tumour implants upon uterus, fallopian tubes or ovaries
T2b (IIB): Tumour extension to pelvic tissues.
T3a (IIIA2): Microscopic, extra-pelvic peritoneal metastasis with or without retroperitoneal lymph node deposits.
T3b (IIIB): Macroscopic, extra-pelvic peritoneal metastasis ≤ 2 cm with or without retroperitoneal lymph node deposits.
T3c (IIIC): Macroscopic,extra-pelvic peritoneal metastasis > 2 cm with or without retroperitoneal lymph node metastasis or tumour extension into liver or splenic capsule without parenchymal incrimination
Regional lymph nodes as common iliac, external iliac, internal iliac, hypogastric, obturator, para-aortic, pelvic or retroperitoneal nodes
NX: Regional lymph nodes cannot be assessed
N0: No regional lymph node metastasis
N0(i+): Isolated tumour cells within regional lymph node(s) ≤ 0.2 mm
N1a (IIIA1i): Lymph node metastasis > 0.2 mm to ≤ 10 mm
N1b (IIIA1ii): Lymph node metastasis > 10 mm
Distant metastasis
M0: No distant metastasis
M1a (IVA): Pleural effusion with malignant cells
M1b (IVB): Liver or splenic parenchymal metastases, deposits within extra-abdominal organs or lymph nodes, inguinal lymph nodes transmural intestinal involvement (3,4).
Ovarian carcinoma can be appropriately discerned with physical or pelvic examination, CA-125 levels and transvaginal ultrasonography. A fixed, nodular, irregular, solid or bilateral adnexal mass may indicate ovarian carcinoma. Pregnancy can be excluded with serum β-HCG levels (3,4). Upon ultrasonography, enlarged, multilocular adnexal mass exhibits papillary configurations, centric vascular articulations and irregular internal septations (3,4). Plain radiographs are beneficial in discerning pleural effusion or metastasis within thoracic cavity (3,4). Computerized tomography (CT) or magnetic resonance imaging (MRI) can be adopted to detect extent of tumefaction within abdominopelvic cavity (3,4). Surgical intervention is required to inspect abdominal cavity and obtain cogent tumour tissue, peritoneal and abdominal lymph node samples for histological evaluation with pertinent immunohistochemistry (3,4). Presence of malignant cells can be discerned within ascitic fluid or peritoneal washings, a feature applicable towards tumour staging (3,4). Generally, unilateral salpingo-oophorectomy is adopted for low grade, stage I carcinoma arising within an un-ruptured ovary, necessitating preservation of fertility. Postmenopausal women with carcinomas of low malignant potential can be subjected to hysterectomy with bilateral salpingo-oophorectomy (3,4). Appendicectomy is required for tumour staging (3,4). Advanced mucinous ovarian carcinomas (stage III or stage IV), extensive peritoneal metastasis or carcinomatous deposits within diaphragm, transverse fissure of liver, mesentery or significant ascites necessitate cyto-reduction or de-bulking (3,4). ‘Interval debulking’ is a protocol where pre-operative, neoadjuvant chemotherapy is followed by debulking surgery and terminated with pertinent chemotherapeutic regimen (3,4). Omentectomy, splenectomy, bowel resection, resection of diaphragm, appendicectomy or posterior pelvic exenteration may be additional treatment measures (3,4). Reoccurrence of ovarian carcinoma necessitates secondary surgical procedures (3,4). Hormone replacement therapy can be adopted in younger subjects with borderline or invasive ovarian carcinoma as singular administration of oestrogen or combined oestrogen and progesterone(3,4).Immunotherapy with bevacizumab can be employed for incompletely resected or stage IV neoplasms (3,4). Commonly, platinum-based drugs such as paclitaxel, cisplatin, topotecan, doxorubicin, epirubicin or gemcitabine are administered. Agents such as olaparib, vincristine, dactinomycin, cyclophosphamide or oxaliplatin are beneficial in reoccurring malignancies or tumefaction resistant to platinum-based therapy. Carboplatin is combined with paclitaxel (3,4). Radiotherapy is utilized for treating stage I and stage II carcinomas or as a palliative therapy in advanced malignancies (3,4). Palliation of terminal clinical symptoms or complications as pain, nausea, constipation, ascites, gastrointestinal obstruction, oedema, pleural effusion and mucositis is necessitated (3,4). Advanced mucinous adenocarcinoma is exceptional, resistant to platinum-based chemotherapy and exhibits an inferior prognosis (3,4). Ovarian carcinoma undergoes capsular rupture, exfoliates and metastasizes within abdominal cavity with tumour deposits upon surface of abdominal viscera, omentum, peritoneum, regional lymph nodes, infundibulo-pelvic ligament, broad ligament or round ligament (3,4). Commonly, metastasis occurs within para-aortic, hypogastric, external iliac, obturator or inguinal lymph nodes. Distant metastasis into brain, hepatic, pulmonary or renal parenchyma is absent (3,4). Factors which enhance prognostic outcomes are absence of residual disease following surgery (stage III or stage IV), complete macroscopic tumour resection (stage IV),age of implicated individuals <45 years, non-serous ovarian carcinoma, tumours with BRCA2 mutation or low histologic grade, preliminary tumour stage, concurrence with endometrial carcinoma and decimated CA-125 values (3,4). Factors associated with inferior prognostic outcomes are rupture of ovarian capsule during surgery, elderly subjects > 45 years, mucinous or clear cell subtype, stage IV disease, enhanced histologic grade, elevated CA-125 or cyclooxygenase 2 levels, tumour dissemination into upper abdominal region or haematogenous dissemination (3,4). Mucinous neoplasms devoid of stromal invasion and borderline or malignant lesions confined to ovary exhibit ≥ 90% 10 year proportionate survival whereas invasive mucinous cystadenocarcinomas demonstrate a survival percentage of ~ 30% (3,4). An estimated 20% of stage I and stage II neoplasms display reappearances, predominantly abdominal, within 5 years of therapy (3,4).
References
- Hada T, Miyamoto M, Ishibashi H, Matsuura H, Kakimoto S, et al. (2022) Comparison of clinical behavior between mucinous ovarian carcinoma with infiltrative and expansile invasion and high-grade serous ovarian carcinoma: a retrospective analysis. Diagn Pathol. 17(1):1-10.
- Armstrong DK, Alvarez RD, Bakkum-Gamez JN, Barroilhet L, Behbakht K, et al. (2019) NCCN guidelines insights: ovarian cancer, Version 1. 2019. J Natl Compr Canc Netw. 17(8):896-909.
- Peres LC, Cushing-Haugen KL, Köbel M, Harris HR, Berchuck A, et al. (2019) Invasive epithelial ovarian cancer survival by histotype and disease stage. J Natl Cancer Inst. 111(1):60-84.
- Perren TJ. (2016) Mucinous epithelial ovarian carcinoma. Ann Oncol. 27:i53-7.

