The Pestilential Pallium-Mantle Cell Lymphoma
Anubha Bajaj*
Department of Histopathology, AB Diagnostics, New Delhi, India
*Corresponding author: Bajaj A, Department of Histopathology, AB Diagnostics, New Delhi, India.
Citation: Bajaj A. (2022) The Pestilential Pallium- Mantle Cell Lymphoma. Genesis J Surg Med. 1(2):1-06.
Received: October 6, 2022 | Published: October 24, 2022
Copyright© 2022 Genesis Pub by Bajaj A. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC BY 4.0). This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author(s) and source are properly credited.
DOI: https://doi.org/10.52793/GJSM.2022.1(2)-8
Abstract
Mantle cell lymphoma is a mature B cell lymphoma composed of miniature to intermediate, atypical, monop-morphic lymphoid cells. The neoplasm is associated with chromosomal translocation t(11;14)(q13;q32) or IGH/CCND1 along with over expression of cyclin D1 and demonstrates clinically aggressive behaviour. Terminology such as centrocytic lymphoma, lymphocytic lymphoma of intermediate differentiation or mantle zone lymphoma to describe mantle cell lymphoma appears antiquated. Mantle cell lymphoma configures ~10% of non Hodgkin’s lymphomas and ~7% of B cell lymphomas. Median age of disease occurrence is 68 years.
Introduction
A male predominance is observed with male to female proportion of 2 to 3:1[1-2]. Mantle cell lymphoma commonly occurs within lymph node, bone marrow, peripheral blood, spleen or hepatic parenchyma. Besides, gastrointestinal tract, Waldeyer’s ring, pulmonary parenchyma, pleura, central nervous system or diverse cutaneous surfaces are frequently implicated extra-nodal sites [1-2]. Extra-nodal emergence of mantle cell lymphoma in the absence of lymph node incrimination and enlargement appears in ~15% instances. Mantle cell lymphomadelineates chromosomal translocation t[11-14] along with IGH/CCND1 genes which activate progression from G1 to S phase of cell cycle [1-2].
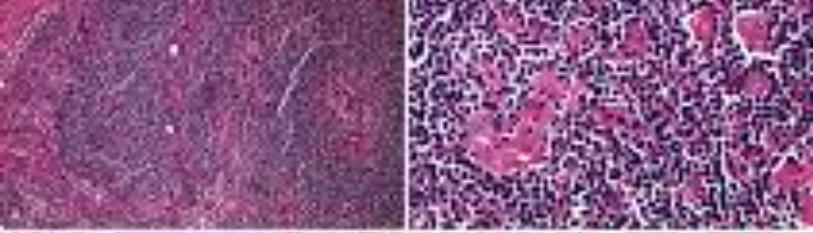
Figure 1: Mantle cell lymphoma composed of monomorphic, atypical lymphoid cells with clumped chromatin and inconspicuous nucleoli with a lack of germinal centres [5].
Figure 2: Mantle cell lymphoma delineating monotonous, atypical lymphoid cells with clumped nuclear chromatin, inconspicuous nucleoli and lack of germinal centres [6].
IGHV gene appears non mutated or minimally mutated in majority of neoplasms. Genetic translocation t[11-14] (q13;q32) may be observed. Fluorescent in situ hybridization (FISH) may be conveniently performed upon fixed tissue sections. FISH may delineate genomic rearrangements of cyclin D2 in instances non reactive to cyclin D1-[1-2]. Fresh tissue samples may be subjected to conventional cytogenetic analysis for discerning the lymphoma. Polymerase chain reaction (PCR) assay of mantle cell lymphoma commonly detects a major ‘breakpoint region [1-2]. Additionally, secondary chromosomal aberrations may appear as ~gains of chromosomes 3q26, 7p21, 8q24 (MYC) or 13q31~lossof chromosomes 1p13-31, 6q23-27 (TNFAIP3), 9p21 (CDKN2A which encodes for p16 INK4a and p14 ARF) 11q22-23 (ATM), 13q11-13, 13q14-34 or 17p13 (TP53) [1-2].
Mantle cell lymphoma demonstrates pre-germinal centre cell of origin with immune reactive SOX11+ and non mutated IGHV gene. Besides, post germinal centre origin with a subset of neoplasms immune non reactive to SOX11- and hyper-mutated IGHV may be exemplified. Majority (70%) of mantle cell lymphomas manifest stage IV disease at initial representation [1-2]. Mantle cell lymphoma exemplifies distinct subtypes as aggressive disease incriminating lymph nodes, immune reactive to SOX11+ indolent disease with leukemic representation, absence of lymph node involvement and immune non reactiveSOX11. Besides, generalized lymphadenopathy, hepatomegaly or splenomegaly may ensue [1-2]. Up to 70% mantle cell lymphomas depict leukemic involvement at initial disease representation. Peripheral blood enunciates atypical lymphoid cells within comprehensive (100%) instances,as discerned by flow cytometry. Multiple intestinal polyps, designated as lymphomatous polyposis may be exemplified [1-2]. Classic mantle cell lymphoma may evolve into blastoid or pleomorphic variant. Aforesaid transformation is encountered in ~one fifth (22%) of relapsed mantle cell lymphoma [1-2].
Cytological examination enunciates a monotonous population of miniature to intermediate lymphoid cells incorporated with distinct, pale to basophilic cytoplasm, nuclear clefts, finely stippled nuclear chromatin and inconspicuous nucleoli [1-2]. Grossly, incriminated lymph node is enlarged. Cut surface is tan, homogenous and demonstrates or may be devoid of indistinct tumour nodules. Spleen enunciates a generalized, micro-nodular configuration with perivascular tumour infiltration [1-2]. Gastrointestinal tract exhibits foci of lymphomatoid polyposis with multiple lymphoid polyps sprinkled upon small and large bowel. Additionally, mucosal ulcers, tumour nodules and diffuse mucosalthickening may be encountered. Microscopic infiltration of atypical lymphoid cells within diverse viscera in the absence of a grossly visible lesion is commonly encountered [1-2]. Upon microscopy, incriminated lymph node depicts a diffuse, nodular or mantle zone tumour configuration, in decreasing order of frequency. Nodal mantle cell lymphoma appears nodular in> 50% instances whereas diffuse mantle cell lymphoma is nodular in < 50% tumefaction.
Neoplastic nodules are devoid of proliferation centres. Mantle cell lymphoma is composed of monomorphic, atypical lymphoid cells of miniature to intermediate magnitude. Tumour cells display clumped nuclear chromatin, inconspicuous nucleoli and irregular nuclear perimeter [1-2]. Generally, neoplastic cellular component is devoid of centroblasts, immunoblasts or para-immunoblasts. Foci of epithelioid histiocytes may be discerned. Vascular articulations appear hyalinised [1-2]. Mantle cell lymphoma demonstrates distinct subcategories as aggressive variant denominates blastoid mantle cell lymphoma is a monomorphic neoplasm appearing aslymphoblast-like and simulates lymphoblastic lymphoma. Mitotic activity is significant and occurs as >20mitosis to 30 mitoses per 10 high power fields [1-2].
Results and Discussions
Pleomorphic mantle cell lymphoma consists of enlarged cells with abundant pale cytoplasm, encephalolike nuclei with prominent nucleoli, and irregular perinuclear infiltration. Multinucleated cells are observed. This neoplasm is not monomorphic and resembles diffuse large B-cell lymphoma. Non aggressive variant denominates small cell mantle cell lymphoma is composed of miniature, spherical lymphocytes pervaded with clumped nuclear chromatin, akin to chronic lymphocytic leukaemia [1-2]. Marginal zone-like mantle cell lymphoma is comprised of lymphoid cells permeated with abundant, pale cytoplasm. Neoplasm simulates marginal zone or monocytoid B cells. Few instances depict foci of lympho-plasmacytic differentiation. Follicular dendritic cell (FDC) meshwork enunciates a nodular configuration with primary follicle-like pattern, germinal centre-like pattern or diffuse pattern [1,2]. ~bone marrow infiltrate of mantle cell lymphoma manifests as nodular, interstitial, para-trabecular or an amalgamation of aforesaid tumour configurations. Peripheral blood demonstrates neoplastic configuration akin to tissue samples. Tumour cell nucleoli may be prominent [1-2]. Spleen in filtrated by mantle cell lymphoma delineates tumour nodules confined to enlarged white pulp along with variable incrimination of red pulp. A residuum of naked germinal centres may ensue. Tumour cells appear monotonous. Foci of marginal zone-like appearance may be discerned in specific instances. Gastrointestinal tract may simulate lympho-epithelial lesions emerging within marginal zone lymphoma [1-2]. Relapse within mantle cell lymphoma enunciates decimated configuration of mantle zone. Neoplastic cells exhibit pleomorphic nuclei of increased magnitude and well dispersed nuclear chromatin. Mitotic activity and Ki67 proliferation index is enhanced. Blastoid mantle cell lymphoma may relapse with classic morphology [1-2] in table 1 and 2.
Mantle cell lymphoma international prognostic index is composed of clinical parameters as age, performance status as per Eastern Cooperative Oncology Group (ECOG), serum lactate dehydrogen as (LDH) levels and leukocyte count. Thus contemplated, three morphologic groups manifest with significantly divergent prognostic outcomes. Mantle cell lymphoma prognostic index (MIPI) score is suitably adopted to stratify outcomes of neoplastic metamorphosis and progression and is constituted of
•age wherein increasing age is accompanied by unfavourable prognosis.
•serum lactate dehydrogenase (LDH) level is indicative of cellular injury wherein elevated levels are beneficial in detecting mantle cell lymphoma.
•Eastern Cooperative Oncology Group (ECOG) performance status appropriately assesses functional activity of incriminated subjects.
•leukocyte count within peripheral blood is evaluated as 10³cells/microliter or 10³cells/µL or 10⁹ cells/litre.
•Ki67 is a cellular marker protein associated with cellular proliferation wherein Ki67 proliferative index in percentage is indicative of neoplastic growth fraction and progression and appears as a component of MIPIb.
Calculation of MIPI is obtained as
MIPI formula = [0.03535 × Age (years)] + 0.6978 (if ECOG 2-4) + [1.367 × log10 (LDH/ULN)] + [0.9393 × log10(WBC)]
ULN (Upper Limit Normal of LDH), WBC (White Blood Cell Count per microliter or x106).MIPI is stratified as •low risk lymphoma with score between 0 to 3
•intermediate risk lymphoma with score between 4 to 5.
•high risk lymphoma with score between 6 to 11
|
MIPI score |
Disease risk |
Median overall survival |
|
<5.7 |
Low |
Not attained |
|
5.7 to <6.2 |
Intermediate |
51 months |
|
≥6.2 |
High |
29 months |
Table 1: International Prognostic Index score for mantle cell lymphoma [2-3].
|
MIPIb score |
Disease risk |
Median overall survival |
|
<5.7 |
Low |
Not attained |
|
5.7 to <6.5 |
Intermediate |
58 months |
|
≥6.5 |
High |
37 months |
Table 2: Biological International Prognostic Index for mantle cell lymphoma [2-3].
References
- Lynch DT, Koya S, Acharya U, Kumar A. (2022) Mantle Cell Lymphoma. StatPearls International 2022, Treasure Island, Florida.
- Pu JJ, Savani M, Huang N, Epner ME. (2022) Mantle cell lymphoma management trends and novel agents: where are we going? Ther Adv Hematol. 13:20406207221080743.
- Tarockoff M, Gonzalez T, Ivanov S, Sandoval-Sus J. (2022) Mantle Cell Lymphoma: the Role of Risk-Adapted Therapy and Treatment of Relapsed Disease. Curr Oncol Rep. 24(10):1313-26.
- Bond DA, Martin P, Maddocks JK. (2021) Relapsed Mantle Cell Lymphoma: Current Management, Recent Progress, and Future Directions. J Clin Med. 10(6):1207.
- Image 1 Courtesy: Libre pathology.
- mage 2 Courtesy: Lancet oncology.

