Delfin Lovelina Francis1*, Saravanan Sampoornam Pape Reddy2, Rangarajan Harikrishnan3, Shreehari Ambika Krishnan4, Sukbhir Singh Chopra5
1Professor, Department of Public Health Dentistry Saveetha Dental College and Hospital, SIMATS, Chennai, Tamil Nadu, India
2Assistant Professor, Department of Periodontology Army Dental Centre (Research & Referral), Dhaula Kuan, Delhi, India
Assistant Professor, Department of Oral & Maxillofacial Surgery Army Dental Centre (Research & Referral), Dhaula Kuan, Delhi, India
4Associate Professor & Head, Department of Periodontology Army Dental Centre (Research & Referral), Dhaula Kuan, Delhi, India
5Commandant, Professor & Head, Department of Orthodontics & Dentofacial Orthopaedics Army Dental Centre (Research & Referral), Dhaula Kuan, Delhi, India
*Corresponding author: Delfin Lovelina Francis, Professor, Department of Public Health Dentistry Saveetha Dental College and Hospital, SIMATS, Chennai, Tamil Nadu, India.
Citation: Francis DL, Reddy SSP, Harikrishan R, Krishan SA, Chopra SS. (2023) The Hidden Threat of Third and Fourth-Hand Smoking to Oral and Periodontal Health – A Narrative Review. J Oral Med and Dent Res. 4(2):1-12.
Received: August 02, 2023 | Published: August 28, 2023
Copyright© 2023 genesis pub by Francis DL, et al. CC BY-NC-ND 4.0 DEED. This is an open-access article distributed under the terms of the Creative Commons Attribution-Non-Commercial-No Derivatives 4.0 International License., This allows others distribute, remix, tweak, and build upon the work, even commercially, as long as they credit the authors for the original creation.
DOI: http://doi.org/10.52793/JOMDR.2023.4(2)-41
Abstract
The act of smoking has been widely recognized as a significant risk factor for the development of different systemic disorders, which can have detrimental effects on both the oral cavity and periodontium. A comprehensive examination of the literature pertaining to the correlation between passive smoking and periodontal disease indicates a substantial incidence of this condition among individuals who do not actively smoke but are exposed to second-hand smoke.
Third-hand smoking is the term used to describe the residual cigarette smoke that remains on various surfaces, such as garments and furniture, for an extended period of time. The principal public health risk related with this issue pertains to the potential for exposing non-smokers, particularly vulnerable populations such as children and the elderly, to detrimental chemicals. More than 6500 hazardous chemicals, including carcinogens and poisons, have the potential to be absorbed through the skin, consumed, or inhaled as a result of exposure to third-hand smoke. Hence, passive smoking involves not alone the inhalation of second-hand smoke, but also the exposure to third- and fourth-hand smoke, thereby potentially contributing to an elevated incidence of periodontal diseases. As a result, it is imperative to provide the general population with education on protecting their oral health through the cessation of smoking and the avoidance of exposure to second- and third-hand smoke, which can be achieved by implementing smoke-free environments.
Keywords
Smoking; Passive smoking; Third hand smoking; Oral effects; Periodontal
Introduction
Smoking is often thought of as a personal decision with repercussions, but there's a hidden danger that many fails to take into consideration: "Second and Third-hand Smoking". Tobacco contains over 6500 components that have been discovered. Particulate matter was frequently assigned more weight in tobacco health hazard research. Nicotine is the most frequently attributed component of all. One of the riskiest vices anyone can have is smoking. It may result in a number of health issues, including heart disease, asthma, and lung cancer. Tobacco smoking's effects on oral and periodontal tissues have been thoroughly studied over decades, and it has been proven as a validated risk factor leading to the initiation, progression, and poor prognosis of healing outcomes [1]. With the introduction of the newer classification of periodontal and peri-implant diseases by the American Academy of Periodontology in 2017, smoking was substantially confirmed as a 'grade modifier,' which means that it can accelerate disease progression from one severity level to the next [2]. Second hand smoking (SHS) also known as passive smoking, is the term used to describe when someone inhales smoke from a cigarette's burning end or smoke that a user exhales [3]. Third hand smoking (THS) the invisible and odourless residue from smoking that lingers in fabrics and on surfaces which has been linked to numerous serious health risks for both adults and children and is a serious public health concern which is often neglected and even omitted in clinical tobacco research [4]. However, few are aware of the danger it can pose to oral health, particularly periodontitis, an immunoinflammatory disease affecting the periodontium that leads to attachment loss and tooth mortality. Unfortunately, THS can cause a variety of systemic and oral health problems, the most serious of which is periodontitis. We can reduce the chance of serious oral health issues by understanding the link between THS and periodontitis and taking the required precautions to protect individuals from its effects. In this article, we discuss the relationship between THS and periodontal diseases, as well as how we can best safeguard our patients from it.
Search Strategy and Selection Criteria
PubMed searches were conducted from 1995 through June 2023, using the terms "third hand smoking," "second hand smoking," "smoking," and "periodontal diseases" to identify relevant references for this study. Articles were also found by searching the writers' personal archives. Only papers written in English were considered for this evaluation. References were selected for inclusion in the final list based on their uniqueness and their applicability to the broader purview of this Review.
Third Hand Smoking and Periodontal Diseases
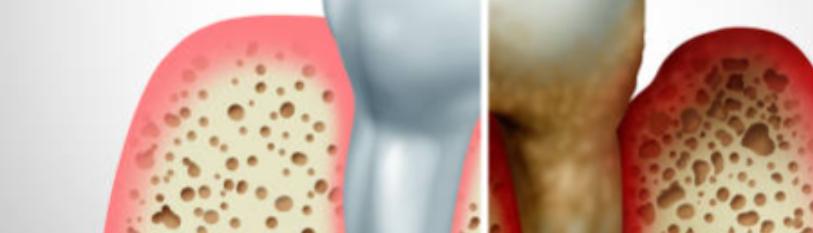
THS is a term used to describe the lingering and residual tobacco smoke and associated chemicals that are found in air, dust, carpets, and furniture as well as on surfaces long after a cigarette has been extinguished [Figure 1]. It is a silent killer that can have a devastating impact on the health of those who are exposed to it, particularly those with weakened immune systems [5]. This invisible, odourless residue can be ingested, inhaled, or even absorbed through the skin, and has been linked to a variety of medical conditions, including periodontitis. Smoking is currently accepted as a ‘grade modifier’ for the classification of periodontal disease after diabetes mellitus. To understand the risks of third-hand smoke, it is important to understand the relationship between smoking and periodontitis.
Insufficient evidence suggests that being around third-party smokers may increase the risk of developing periodontitis. However, the compounds in tobacco smoke can inflame and irritate the gingiva, increasing their susceptibility to infection. In addition, a number of studies have demonstrated that THS chemicals can affect oral tissues and alter the oral microbial equilibrium, both of which can accelerate the onset of periodontitis [6]. The dangers of SHS as a public health concern are well documented and extensively discussed, whereas the dangers of THS are frequently overlooked. It is the third leading cause of mortality in the United States, after tobacco use and alcohol consumption. In developing nations like India, the same health hazard is not accorded the importance it deserves in South Asian nations, which are more ignorant and careless [7]. In reality, the innocent subjects are exposed to quantitatively lesser and qualitatively distinct levels of SHS and THS. This grave public health concern can cause periodontitis, gingival recession, and even tooth loss due to the toxins in THS, which can trigger an inflammatory response and contribute to gingivitis, gingival recession, and tooth loss [8] Consequently, it is essential to comprehend the risks associated with THS and to take measures to limit exposure as a potential public health hazard. However, there is an additional, often unseen risk associated with smoking: the risk of developing periodontitis due to exposure to third-hand smoke. Consequently, it is essential to be aware of the dangers posed by THS and how it can cause periodontitis [Figure 1].
THS and Gingival Bleeding
Some evidence suggests that THS exposure may increase the chance of gingival bleeding. Gingival bleeding, which is frequently a symptom of periodontal disease and indicates poor dental health, can occur. An investigation found that non-smokers who were subjected to THS had more gingival bleeding than non-smokers [9]. The research also found a link between THS exposure and an increased risk of periodontal disease. Another research found that thirdhand smoke exposure increased the levels of inflammatory biomarkers in non-smokers' saliva [10]. Inflammation is a major factor in periodontal disease and can result in gingival bleeding [11]. Although more research is required to fully understand the connection between THS and gingival bleeding, these studies indicate that exposure to THS may increase the risk of periodontal disease and gingival bleeding. Therefore, it's essential to restrict exposure to third-party smoke in order to promote optimal oral health.
THS and Periodontitis
Periodontitis was more frequent in non-smokers who were exposed to SHS than in non-smokers. The study found that non-smokers had a higher chance of developing periodontitis than non-smokers who did not live with smokers or who were exposed to SHS [12]. Patients with periodontitis who were also exposed to SHS showed more inflammation and bone loss. SHS was associated with increased levels of inflammation markers in gingival crevicular fluid (GCF) and increased loss of alveolar bone in non-smokers, according to the research [13].
When children are subjected to ambient environmental tobacco smoke (ETS), negative health effects may result. An interesting questionnaire-based study investigated the association between the prevalence of oral health problems including dental caries, gingivitis, mucosal pigmentation and enamel defects in one to five-year-old children exposed and not exposed to environmental tobacco smoke before and/or after birth. It was found that people exposed to ETS had middle ear infections and upper respiratory tract infections during the neonatal period, in addition to having higher caries indices and significantly higher gingival index scores than the non-ETS participants. Those exposed to ETS before and after birth had the highest incidence of enamel opacities and showed a higher risk for dental caries, despite the fact that more children in this group used the recommended fluoride toothpaste. (1000 ppm fluoride). In that study, salivary changes in young infants as well as associations between ETS exposure before and after pregnancy and oral health were examined. This embarks a significant point that dental health professionals should include a question about household smoking in pediatric dental history taking, which would allow opportunities to discuss the impact of SHS / THS on child’s oral health along with definitive longitudinal oral health studies should include a history of maternal smoking during pregnancy and afterwards [14].
According to study that was published very early, tobacco use is unquestionably a risk factor for periodontal disease. It also has an impact on the treatment and outcomes of periodontal disease at different stages [15]. The world's populace has a well-known habit of smoking, and systematic reviews have established a link between smoking and periodontal disease over time. There is a wealth of cross-sectional data to support the association between smoking and periodontitis, but the strength of the association varies based on the periodontal indices and the criteria used to identify periodontitis. The bulk of studies, however, have only looked at how smoking and passive smoking affect periodontal pathogenesis, periodontal microbiology, and the effectiveness of periodontal therapy [16]. The possibility of getting periodontitis increases significantly when one is around second-hand tobacco. People who have been subjected to second-hand smoke are more likely to develop severe periodontitis than people who have not. On the other hand, a Japanese study discovered a connection between active smoking and a greater prevalence of periodontal disease in young women, but not passive smoking [17]. There has been no research into the impacts of THS on periodontal tissues, but this is likely because of the lack of knowledge surrounding THS. This may be one of the reasons that contribute to the failure of periodontal therapy as the iceberg continues to melt. The number of cigarettes smoked considerably increases the risk of implant failure, according to a meta-analysis examining the relationship between smoking and implant failures [18]. Despite optimal clinical conditions, many implant failures occur in systemically healthy, non-smoking patients. The role of genetic and epigenetic factors with elevated gingival and bone DNA Methylation levels as potential contributors to such failures is being investigated [19]. It is unclear, however, whether THS contributes to implant complications or early or late implant failure.
It is well-known that smoking causes environmental changes and consequently alters the microbial flora of the oral cavity, which is colonized by more than 700 species, which contributes substantially to the emergence of putative periodontal pathogens [20]. Both active and passive smoking have been shown to increase the number of bacteria and modify their metabolic pathways [21]. In addition, it revealed a significant correlation between upbringing and the presence of potential pathogens such as Prevotella, Veillonella, Atopobium, Megasphaera, and Bullsphaera [22]. It reveals that smoking significantly alters the oxygen availability of microorganisms and their colonies. Cigarette smoking modifies the chemical structure of DNA, resulting in transcriptional alterations, of which fifty percent are caused by epigenetic mechanisms. In almost all cells, including epithelial, glandular, and connective tissue cells, it influences DNA methylation and its metabolites. It has substantial effects on chemotaxis, cell movement, and immune function. During inflammation, the diapedesis and movement of mononuclear leukocytes are crucial processes that are among the most affected [23]. As genetic variations associated with an increased risk of excessive smoking, increased intensity of smoking, and smoking-related disease and mortality, multiple phenotypic/genotypic relationships have been established [24].
Potential Causal Link between Periodontal Diseases and THS
Cigarette smoking has been considered a possible cause of periodontitis. In moderate to heavy smokers, the odds of clinical attachment loss ranged from 3·25 to 7·28%, with a risk ratio of 1·85 (95% confidence interval 1·5-2·2) for periodontitis. In a controlled study with self-reported environmental tobacco smoke exposure and a temporal relationship to environmental tobacco exposure, it was determined that the probabilities of developing periodontal diseases were increased by 1·6 times for those who were exposed to environmental tobacco smoke [25]. Animal studies have also examined the potential effects of third-party tobacco exposure on other dental diseases, such as caries. Exposure to THS increased the severity of caries in rats, most likely by altering the oral microbiome and encouraging the proliferation of cariogenic bacteria [26]. Although some animal studies have examined the potential effects of THS on dental diseases, it is important to consider that these results may not always apply to humans. These findings must be confirmed, and additional research is necessary to elucidate the mechanisms by which thirdhand smoke may impair oral health [27]. In another study where mice exposed to thirdhand smoke had more severe periodontitis than mice not exposed. The same study also found that exposure to SHS altered the oral microbiome, which may contribute to the development of periodontitis [28]. Even though these studies provide some evidence of a causal link between THS and periodontitis, additional research is necessary to confirm these results and thoroughly understand the mechanisms by which THS may contribute to periodontal disease.
The cross-sectional data obtained between 1988-1994 from the Third National Health and Nutrition Examination Survey (NHANES) which investigated the relation between ETS and periodontal disease in United States revealed that, exposure to ETS at home only, work only, and both were reported by 18%, 10·7%, and 3·8% of the study population, using questionnaires respectively. In comparison to people who weren't exposed to ETS, the adjusted chances of having periodontal disease were 1·6 (95% confidence interval = 1·1, 2·2) times higher. People in the US who had never used tobacco were more prone to develop periodontal disease than people who had not been exposed to ETS [29]. Such exposure in non-smokers doubles or triples their risk of periodontitis, according to epidemiologic studies using split mouth designs. In response to the possibility of under-reporting of periodontitis, the Centres for Disease Control updated periodontal examination procedures in 2009 for NHANES, including full-mouth, six-site periodontal probing, and attachment loss assessment. A second cross-sectional study using NHANES data from the 2009–2012 examination cycle to estimate periodontitis prevalence in US non-smokers and the effect of ETS exposure. There was a 28% increase in the odds of periodontitis for those with any ETS exposure compared with those with no measurable exposure (Wald χ2 test statistic [df] = 6·58 [1], p = 0·01; 95% confidence interval = 1·06 to 1(55) thereby concluded that ETS exposure increases the risk of an individual developing periodontitis [30]. With the specific query "Is there a relationship between ETS and periodontal disease?" as its focus, a systematic review investigated the association between ETS) and periodontal disease. Nine clinical trials and four in-vitro studies were part of the chosen studies. Five studies found that people exposed to ETS had substantially higher odds of developing periodontal disease than did controls. (non-smoking individuals unexposed to ETS). This exposure did not appear to be associated with periodontal disease in at least two trials. Therefore, the link between ETS and gum disease is still up for debate and needs more research [31]. The occurrence of peri-implantitis in smokers is a well-established fact, with cumulative smoking exposure and a shortened smoking cessation period being directly associated with an increased risk. To reduce the negative effects of cumulative smoking exposure, which includes SHS and THS, and to investigate the positive effects of smoking cessation, general health services must implement educational and preventative strategies. In a 6-year longitudinal periodontal maintenance therapy (PMT) trial, total smoking exposure and smoking cessation duration were examined on periodontitis recurrence. Non-smokers had 44·2%, past smokers 68·2%, and chronic smokers 80% periodontitis recurrence. After adjusting for confounders, odds ratios (95% confidence interval) for recurrence at 6 years were 2.80 (2·11 to 5·14) for past smokers and 5·97 (3·58 to 9·88) for chronic smokers. Cumulative smoking exposure, including SHS and THS, and shorter time since smoking cessation were substantially associated with periodontal disease recurrence after 6 years of PMT [32].
Evidence Linking Periodontitis and SHS
In a meta-analysis on the topic of SHS exposure and periodontitis, which suggests that passive smoking may increase the risk of periodontitis. The researchers found that among 10,171 participants in 13 trials, SHS exposure was associated with a 1·36-fold increased risk of periodontitis compared to no exposure. It was discovered that non-smokers exposed to SHS had higher levels of inflammation markers in their GCF and greater alveolar bone loss than non-smokers not exposed to SHS. There is mounting proof that people who are exposed to SHS are more likely to develop periodontitis and other oral health problems. As a consequence, reducing your contact with SHS is essential for preventing periodontitis and other health problems [33].
The evidence that there is a link between THS and gingivitis is not strong. Despite some evidence that thirdhand smoke may be harmful to health, especially in children, there is little study on the effects of it on periodontitis. The research found that rats subjected to thirdhand smoke lost more bone mass than which were not exposed to THS. It is important to keep in mind that human exposure to third-party smoke may not always have the same effects as those observed in animal research. Another investigation into the relationship between THS and children's periodontal health. Children who were exposed to THS had higher levels of plaque and gingival inflammation than those who were not, according to the research. The study did not, however, explicitly examine the connection between THS and periodontitis. Although there is little evidence connecting THS to periodontitis, the study indicates that it may have negative effects on oral health and contribute to inflammation and bone loss. More study is necessary to fully understand how THS exposure affects periodontitis and other outcomes associated with human oral health [34].
Oral Microbial Changes in THS
There is limited evidence to indicate that THS can alter the composition of the oral microbiome, a community of microorganisms that lives in the mouth. Periodontal disease, dental caries, and halitosis are just a few of the oral health conditions that have been linked to changes in the oral microbiome. A 2019 research found that exposure to THS altered the oral microbiomes of mice. According to the study, mice who had been exposed to thirdhand smoke had higher concentrations of harmful bacteria and lower concentrations of helpful bacteria than mice who had not [35]. While more research is needed to fully understand the relationship between THS and oral microbiome changes, these studies suggest that exposure to thirdhand smoke may alter the balance of microorganisms in the mouth, which may contribute to the development of oral health conditions. Therefore, it's essential to restrict exposure to THS in order to promote optimal oral health [36].
Wound Healing and THS
THS may have a detrimental effect on the outcomes of wound healing. THS can result in the inhalation of toxic chemicals or the absorption of these substances through the epidermis, which can impede the body's normal healing processes. For instance, a recent research discovered that mice's ability to heal wounds was hampered by exposure to THS. The research found that mice who were exposed to thirdhand smoke had more inflammation and slower wound healing than mice who weren't. The researchers found that exposure to THS may conflict with the body's typical immune reaction to injury, which can impede the healing process [37]. In a different study performed in patients having wound healing procedures, it was discovered that exposure to third-hand smoke increased the risk of complications. The research found that THS exposure increased the risk of infection, slowed healing, and other complications in patients compared to those who were not exposed. Although more research is required to fully understand the connection between THS and wound healing outcomes, the findings of these studies indicate that THS may impact the normal course of healing and increase the risk of complications [38]. Therefore, it's crucial to restrict exposure to THS in order to promote the finest results for wound healing.
Oral Malignancies and THS
THS / SHS can have serious health consequences, including lung cancer. More studies are needed to completely understand the link between THS and oral cancer, as evidence for this is still emerging. Exposure to second-hand smoke increases the chance of lung cancer in mice [39]. The research found that mice exposed to third-hand smoke had significantly more DNA damage in their lungs, which could promote the development of cancer [40] Exposure to second-hand smoke has been already linked to an increased risk of lung cancer [41]. Although these studies suggest a link between third-hand smoke and oral cancer, more study is needed to confirm these results and completely understand the underlying mechanisms by which THS may affect oral cancer risk [42]. It's crucial to remember that smoking causes almost all instances of head and neck cancer, and that quitting is the best way to lessen that risk. Estimating the exact incidence of upper aerodigestive cancers due to THS is complicated by the fact that it is often difficult to disentangle the effects of THS from those of other factors that can cause lung cancer, such as exposure to SHS or air pollution [43]. However, research has shown that being around SHS or THS may increase the chance of developing lung cancer, especially in vulnerable populations like young children and non-smoking adults.
It was studied that passive smoking is responsible for 1-5% of lung cancer diagnoses in the United States [44]. Research used a modelling method to assess the potential effect of third-party smoke on lung cancer risk, based on data on the prevalence of third-party smoke exposure and the potential carcinogenicity of the chemicals found in third-party smoke. Although this estimate is uncertain and may change based on the population and area studied, it highlights the potential importance of addressing thirdhand smoke as a public health problem [45]. The risk of lung cancer and other harmful effects of tobacco smoke can be reduced by limiting exposure to thirdhand smoke, particularly among non-smokers and young people.
Emerging Danger of Fourth-Hand Smoke (FHS)
‘Fourth-hand smoke’ in contrast to SHS and THS, refers to the lingering tobacco smoke and related pollutants that are carried preferably on smoker's skin, hair, and clothing. Those who are exposed to smokers, either through direct interaction or by coming into contact with their clothing, may ingest or inhale the smoke that has been released into the air. Some studies have suggested that FHS could be harmful, despite the fact that it is still a novel concept and there hasn't been a lot of study on its effects on health. Exposure to third- and fourth-hand smoke residues on surfaces was associated with increased levels of nicotine in the urine of non-smokers [46]. In a separate research, mice that were exposed to FHS showed increased oxidative stress. However, more study is needed to completely understand the negative effects of fourth-hand smoke exposure on human health. Smokers should avoid close quarters, cleanse their hands frequently, and cover their nose and mouth while waiting to reduce their risk of spreading FHS [39], [47].
Benefits of Smoking Cessation
With the proper knowledge, smokers and mainly those around them can protect their health from SHS/THS by taking the necessary precautions. Despite the fact that these studies suggest a link between SHS/THS and periodontitis, additional research is necessary to fully comprehend this association and identify the underlying mechanisms by which they may contribute to the development of periodontitis. To reduce the risk of periodontitis and other adverse health outcomes, it is essential to not only limit active smoking but also limit SHS/THS, given the known negative health effects of tobacco smoke on oral tissues. Although the negative effects of tobacco on periodontal tissues have been extensively reported, little is known about the potential positive effects of quitting smoking on periodontal health. A systematic review examined prospective studies comparing progression rates of periodontitis between smokers and ex-smokers, as well as clinical trials evaluating the efficacy of smoking-cessation programs alone or in conjunction with periodontal treatment. It was found that quitters were more likely than non-quitters/oscillators to have periodontal probing depth reductions [48]. In addition, a meta-analysis revealed that quitting smoking reduced the risk of periodontitis onset and progression, and enhanced the efficacy of periodontal therapy. After quitting smoking, the risk of periodontitis becomes comparable to that of never-smokers, and the effectiveness of nonsurgical periodontal treatment improves. As an important component of periodontal therapy, dental professionals should consider smoking cessation interventions [49]. A systematic review of smoking cessation behavioural interventional models, namely the transtheoretical model (TTM) and the health belief model (HBM), revealed that the majority of studies lacked participant blinding, thereby increasing the risk of bias in the outcome. Both HBM- and TTM-based trainings were found to have positive effects on smoking cessation and stage progression [50]. However, studies incorporating behavioural interventions in primary care may decrease the likelihood of smoking initiation in non-smoking children and adolescents. Smoking initiation may be reduced by behavioural interventions in non-smoking children and teenagers. However, in order to prevent the effects of SHS/THS, life style modification is advised for vulnerable younger age groups [51]. According to a Cochrane review, there is evidence of moderate certainty that neither reduction-to-quit nor abrupt ceasing interventions result in superior long-term quit rates when compared to each other [52].
Preventive Measures
The most effective way to prevent periodontitis is to practice good oral hygiene, which entails brushing and flossing at least twice per day and visiting the dentist frequently for check-ups and cleanings. In addition, abstaining from smoking and limiting your exposure to SHS/THS can protect your general health and oral hygiene. There is limited evidence that SHS affects periodontal tissues. Although some studies have suggested a potential link between exposure to THS and an increased risk of periodontitis, the evidence remains inconclusive. Numerous negative health outcomes, including cancer, cardiovascular disease, and respiratory issues, have been linked to exposure to both SHS and THS. It can have long-term effects, particularly for young infants who are exposed. Therefore, THS should be avoided [53]. Providers of health care play a crucial role in reducing the peril of thirdhand smoking. Fathers and heavy smokers are generally less prone to view THS as dangerous [54]. If smokers believed that THS was harmful to children's health, they were more likely to restrict smoking in their homes and vehicles. Conversations with smokers about the effects of THS can provide additional justification for promoting smoking cessation and preventing non-smokers’ exposure to cigarette smoke and THS [55]. Self-care is the primary motivation for quitting smoking. However, a review of long-term research revealed that the effect of smoking on family members was a common incentive to cease [56].
One of the Sustainable Development Goals (SDGs – Goal 3) established by the United Nations Development Program (UNDP) for the international community is to ensure that children have access to decent living conditions, including a reduction in infant mortality [57]. In 2003, the WHO Member States adopted the Framework Convention on Tobacco Control (FCTC), emphasizing the obligations of governments to design and implement effective tobacco control programmes. The FCTC establishes rules to protect present and future generations from the negative effects of tobacco use and exposure to tobacco smoke on their health, as well as their social, environmental, and economic well-being. There is substantial evidence that smoking increases infant mortality; therefore, reducing involuntary exposure to smoking contributes to achieving this goal [27]. Strengthening FCTC implementation is one of the SDGs promoted by UNDP. The overlap between the two objectives highlights the need for continuous research on THS in children in order to generate information that will enable governments to intensify their fight against the tobacco epidemic and enhance living conditions [58]. Given that most nations do not prohibit smoking in shared spaces with non-smokers, educating smokers about the dangers of SHS to their families may be an effective strategy for persuading them to cease smoking in these settings [59]. The introduction of explicit regulations prohibiting the use of tobacco products in private residences is improbable or even impossible (due to ethical considerations regarding individual freedom); therefore, education and increasing knowledge about the effects of THS may be advantageous strategies [60]. The effects of THS on health, especially the health of children, should be the focal point of family-centred programs [61].
As THS residues cannot be eliminated by ventilation alone and are often conveyed through clothing, it may be prudent to take precautionary measures such as: [62]
1. Do not smoke inside your residence or vehicle.
2. Do not allow smoking near you, your children, or your pets. In unavoidable situations; i.e., if a person has been in close proximity to a smoker, it is advised to immediately sanitize all personal apparel. Because clothing residue can transfer to other surfaces, one should take a full ablution before moving around the house. If someone has smoked in the residence, all bed sheets, blankets, pillowcases, curtains, etc. should be thoroughly washed. This will ensure that no cancer-causing substances remain in the home.
3. The vapor or aerosol emitted by an electronic cigarette contains chemicals. Do not allow the use of electronic cigarettes in the presence of minors, animals, or in vehicles.
4. Clean the hard surfaces, such as the floors, walls, tabletops, and countertops, with both moist and dry disinfecting solutions.
5. Even on the child's objects, third-hand smoke residue can accumulate, posing a health risk. As these items are high-contact surfaces for children, it is recommended that you clean and disinfect them thoroughly [63].
6. Professional painters have used Trisodium Phosphate (TSP) to eliminate THS residues. Despite being a dangerous chemical, the effects and safety over the long term are still inconclusive [64].
7. Check the smoking histories of prospective local residents. Determine whether new domestic items and furniture were sourced from a smoke-free environment [65].
8. Only quitting will provide total protection against thirdhand smoke. Health care professionals have a substantial obligation to raise public awareness and educate the public about THS prevention measures [66].
Conclusion
Studies of THS exposure in residences have uncovered alarmingly high levels of nicotine and other aromatic carbon compounds. Nonetheless, even within the medical and dental communities, comprehension is limited. As both smokers and non-smokers, the most disadvantaged members of society endure the brunt of the health risks associated with tobacco use due to the lack of legal enforcement. The duration of THS effects in vitro and in vivo remains unknown. Numerous nations already employ ozonation as a standard technique for THS management without completely appreciating the risks involved. In addition, there are no validated biomarker assays for detecting exposure before it occurs. Due to the magnitude and scope of the problem, future research into THS should prioritize next-generation risk assessment through intensively conducted exposure studies. THS is the invisible threat that few people consider. It is the smoke residue that remains on surfaces, clothing, and in the air after smoking a cigarette. Recent research indicates that THS can cause periodontitis, a severe and potentially fatal disease. The majority of people believe that third-hand smoking is innocuous. This is why it is crucial to be aware of the hidden dangers of SHS or THS and to take precautions to safeguard yourself and your family. From understanding the risks to mitigating exposure, this article examines the various ways in which THS can cause periodontitis and the measures you can take to reduce the risks. The ‘WHO Tobacco Free Initiative’ monitors the status of the global tobacco epidemic and provides reports on anti-smoking interventions and policies. There is support of evidence for a hidden periodontitis epidemic driven by smoking in addition to the unknown status of SHS and THS that might have occurred during the 20th century due to late recognition of tobacco smoking and non-recognition of SHS, THS and even FHS as a putative risk factor for periodontitis despite the advances in understanding of risk factors in periodontal diseases. Of interest is the observation that the relative attributable burden of smoking related periodontal diseases is under estimated and more research is needed to combat the effects of SHS, THS and FHS by the whole dental and medical community to be actively involved in smoking cessation and anti-tobacco campaigns.
References
1. Chaffee BW, Couch ET, Vora MV, Holliday RS. (2021) Oral and periodontal implications of tobacco and nicotine products. Periodontol 2000. 87:241-53.
2. Tonetti MS, Greenwell H, Kornman KS. (2018) Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J Periodontol. 89:159-72.
3. Zheng Y, Ji Y, Dong H, Chang C. (2018) The prevalence of smoking, second-hand smoke exposure, and knowledge of the health hazards of smoking among internal migrants in 12 provinces in China: a cross-sectional analysis. BMC Public Health. 24:655.
4. Acuff L, Fristoe K, Hamblen J, Smith M, Chen J. (2016) Third-Hand Smoke: Old Smoke, New Concerns. J Community Health. 41:680–7.
5. Lovelina Francis D, Sampoornam Pape Reddy S. (2022) The silent assassin: Third hand smoking. J Glob Health. 12:03079.
6. Leite FRM, Nascimento GG, Scheutz F, Lopez R. (2018) Effect of Smoking on Periodontitis: A Systematic Review and Meta-regression. Am J Prev Med. 2018; 54:831-41.
7. Glantz SA, Parmley WW. (1996) Passive and active smoking. A problem for adults. Circulation. 94:596–8.
8. Michaud DS, Fu Z, Shi J, Chung M. (2017) Periodontal Disease, Tooth Loss, and Cancer Risk. Epidemiol Rev. 39:49-58.
9. Silva H. (2021) Tobacco Use and Periodontal Disease-The Role of Microvascular Dysfunction. Biology (Basel). 10(5):441.
10. Northrup TF, Khan AM, Jacob P, L.Benowitz N, Hoh E, et al. (2016) Thirdhand smoke contamination in hospital settings: assessing exposure risk for vulnerable paediatric patients. Tob Control. 25:619-23.
11. Goulao B, MacLennan GS, Ramsay CR. (2021) Have you had bleeding from your gums? Self-report to identify giNGival inflammation (The SING diagnostic accuracy and diagnostic model development study). J Clin Periodontol. 48(7):919-928.
12. Sutton JD, Ranney LM, Wilder RS, Sanders AE. (2012) Environmental tobacco smoke and periodontitis in U.S. non-smokers. J Dent Hyg. 86:185-94.
13. Fatima T, Khurshid Z, Rehman A, Imran E, Srivastava KC, et al. (2021) Gingival Crevicular Fluid (GCF): A Diagnostic Tool for the Detection of Periodontal Health and Diseases. Molecule. 26(5):1208.
14. B Hasmun NN, Drummond BK, Milne T, Cullinan MP, Meldrum AM, et al. (2017) Effects of environmental tobacco smoke on the oral health of preschool children. Eur Arch Paediatr Dent. 18(6):393-398.
15. Preber H, Kant T. (1973) Effect of tobacco-smoking on periodontal tissue of 15-year-old schoolchildren. J Periodontal Res. 8(5):278-83.
16. Souto MLS, Rovai ES, Villar CC, Braga MM, Pannuti CM. (2019) Effect of smoking cessation on tooth loss: a systematic review with meta-analysis. BMC Oral Health. 19:245.
17. Sanders AE, Slade GD, Beck JD, Agustsdottir H. (2011) Secondhand smoke and periodontal disease: atherosclerosis risk in communities study. Am J Public Health. 339-346.
18. Naseri R, Yaghini J, Feizi A. (2020) Levels of smoking and dental implants failure: A systematic review and meta-analysis. J Clin Periodontol. 47:518-28.
19. Khouly I, Pardinas Lopez S, Diaz Prado SM, Ferrantino L, Kalm J, et al. (2022) Global DNA Methylation in Dental Implant Failure Due to Peri-Implantitis: An Exploratory Clinical Pilot Study. Int J Environ Res Public Health. 19(2):1020.
20. Sedghi LM, Bacino M, Kapila YL. (2021) Periodontal Disease: The Good, The Bad, and The Unknown. Front Cell Infect Microbiol. 11:766944.
21. Hyde ER, Andrade F, Vaksman Z, Parthasarathy K, Jiang H, et al. (2014) Metagenomic analysis of nitrate-reducing bacteria in the oral cavity: implications for nitric oxide homeostasis. PloS One. 9(3):88645.
22. Jia YJ, Liao Y, He YQ, Zheng MQ, Tong XT, et al. (2021) Association Between Oral Microbiota and Cigarette Smoking in the Chinese Population. Front Cell Infect Microbiol. 11:658203.
23. Ringh MV, Hagemann-Jensen M, Needhamsen M, Kular L, E Breeze C, et al. (2019) Tobacco smoking induces changes in true DNA methylation, hydroxymethylation and gene expression in bronchoalveolar lavage cells. EBioMedicine. 46:290-304.
24. Bierut LJ, Tyndale RF. (2018) Preparing the Way: Exploiting Genomic Medicine to Stop Smoking. Trends Mol Med. 24(2): 187–196.
25. Arbes SJ, Agustsdottir H, Slade GD. (2001) Environmental tobacco smoke and periodontal disease in the United States. Am J Public Health. 91(2): 253-257.
26. Hang B, Wang P, Zhao Y, Sarker A, Chenna A, et al. (2017) Adverse Health Effects of Thirdhand Smoke: From Cell to Animal Models. Int J Mol Sci. 18(5):932.
27. Bahl V, Shim HJ, Jacob P, Dias K, Schick SF, et al. (2016) Thirdhand smoke: Chemical dynamics, cytotoxicity, and genotoxicity in outdoor and indoor environments. Toxicol Vitro Int J Publ Assoc. 32:220-31.
28. Liu H, Liu Z, Meng L, Fu X, Hou Y. (2019) Toxic effects of 1-(N-methyl-N-nitrosamino)-1-(3-pyridinyl)-4-butanal on the reproduction of female mice. Ecotoxicol Environ Saf. 183:109544.
29. Inoue Y, Zaitsu T, Akiko O, Ishimaru M, Taira K, et al. (2021) Association between exposure to secondhand smoking at home and tooth loss in Japan: A cross-sectional analysis of data from the 2016 National Health and Nutrition Survey. Tob Induc Dis.19:96.
30. Sutton JD, Salas Martinez ML, Gerkovich MM. (2017) Environmental Tobacco Smoke and Periodontitis in United States Non-Smokers, 2009 to 2012. J Periodontol. 88:565-74.
31. Javed F, Bashir Ahmed H, Romanos GE. (2014) Association between environmental tobacco smoke and periodontal disease: a systematic review. Environ Res. 133:117-22.
32. Costa FO, Lages EJP, Cortelli SC, Cortelli JR, Mattos-Pereira GH, et al. (2022) Association between cumulative smoking exposure, span since smoking cessation, and peri-implantitis: a cross-sectional study. Clin Oral Investig. 26(7):4835-4846.
33. Vieira TR, Martins CC, Cyrino RM, Azevedo AMO, Cota LOM, et al. (2018) Effects of smoking on tooth loss among individuals under periodontal maintenance therapy: a systematic review and meta-analysis. Cad Saude Publica. 34(9):00024918.
34. Chang CH, Han ML, Teng NC, Lee CY, Huang WT, et al. Cigarette Smoking Aggravates the Activity of Periodontal Disease by Disrupting Redox Homeostasis-An Observational Study. Sci Rep. 8(1):11055.
35. Yu M, Mukai K, Tsai M, Galli SJ. (2018) Thirdhand smoke component can exacerbate a mouse asthma model through mast cells. J Allergy Clin Immunol. 42(5): 1618-1627.
36. Kelley ST, Liu W, Quintana PJE, Hoh E, Dodder NG, et al. (2021) Altered microbiomes in thirdhand smoke-exposed children and their home environments. Pediatr Res 90(6):1153-1160.
37. Villalobos-Garcia D, Ali HEA, Alarabi AB, El-Halawany MS, Alshbool FZ, et al. (2022) Exposure of Mice to Thirdhand Smoke Modulates In Vitro and In Vivo Platelet Responses. Int J Mol Sci. 23(10):5595.
38. Dhall S, Alamat R, Castro A, Sarker AH, Mao JH, et al. (2016) Tobacco toxins deposited on surfaces (third hand smoke) impair wound healing. Clin Sci (Lond). 130(14):1269-84.
39. Adhami N, Chen Y, Martins-Green M. (2017) Biomarkers of disease can be detected in mice as early as 4 weeks after initiation of exposure to third-hand smoke levels equivalent to those found in homes of smokers. Clin Sci Lond Engl. 131:2409-26.
40. Hang B, Wang P, Zhao Y, Chang H, Mao JH, et al. (2020) Thirdhand smoke: Genotoxicity and carcinogenic potential. Chronic Dis Transl Med. 6:27-34.
41. Thandra KC, Barsouk A, Saginala K, Aluru JS, Barsouk A. (2021) Epidemiology of lung cancer. Contemp Oncol Poznan Pol. 25:45-52.
42. Ganjre AP, Sarode GS. (2016) Third hand smoke--A hidden demon. Oral Oncol. 54:3-4.
43. Gupta B, Kumar N, Johnson NW. (2017) Relationship of Lifetime Exposure to Tobacco, Alcohol and Second Hand Tobacco Smoke with Upper aero-digestive tract cancers in India: A Case-Control Study with a Life-Course Perspective. Asian Pac J Cancer Prev. 18:347-56.
44. Carreras G, Lugo A, Gallus S, Cortini B, Fernandez E, et al. (2019) Burden of disease attributable to second-hand smoke exposure: A systematic review. Prev Med. 129:105833.
45. https://www.thelancet.com/journals/eclinm/article/PIIS2589-5370(23)00113-X/fulltext
46. De Granda-Orive JI, Jimenez-Ruiz CA, Solano-Reina S. (2017) World Health Organization positioning. The Impact of Tobacco in the Environment: Cultivation, Curing, Manufacturing, Transport, and Third and Fourth-hand Smoking. Arch Bronconeumol. 30294-6.
47. Chen H, Li G, Allam VSRR, Wang B, Chan YL, et al. (2020) Evidence from a mouse model on the dangers of thirdhand electronic cigarette exposure during early life. ERJ Open Res. 6:00022-2020.
48. Fiorini T, Musskopf ML, Oppermann RV, Susin C. (2014) Is there a positive effect of smoking cessation on periodontal health? A systematic review. J Periodontol. 85:83-91.
49. Leite FRM, Nascimento GG, Baake S, Pedersen LD, Scheutz F, et al. (2019) Impact of Smoking Cessation on Periodontitis: A Systematic Review and Meta-analysis of Prospective Longitudinal Observational and Interventional Studies. Nicotine Tob Res Off J Soc Res Nicotine Tob. 2:1600-8.
50. Ravi K, Indrapriyadharshini K, Madankumar PD. (2021) Application of Health Behavioral Models in Smoking Cessation - A Systematic Review. Indian J Public Health. 65:103-9.
51. Selph S, Patnode C, Bailey SR, Pappas M, Stoner R, et al. (2020) Primary Care-Relevant Interventions for Tobacco and Nicotine Use Prevention and Cessation in Children and Adolescents: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA. 323:1599-608.
52. Lindson N, Klemperer E, Hong B, Ordonez-Mena JM, Aveyard P. (2019) Smoking reduction interventions for smoking cessation. Cochrane Database Syst Rev. 9(9):CD013183.
53. http://www.ncbi.nlm.nih.gov/books/NBK53017/
54. Drehmer JE, Ossip DJ, Rigotti NA, Nabi-Burza E, Woo H, et al. (2012) Pediatrician interventions and thirdhand smoke beliefs of parents. Am J Prev Med. 43:533-6.
55. Pisinger C, Aadahl M, Toft U, Jorgensen T. (2011) Motives to quit smoking and reasons to relapse differ by socioeconomic status. Prev Med. 52:48-52.
56. Williams RJ, Herzog TA, Simmons VN. (2011) Risk perception and motivation to quit smoking: a partial test of the Health Action Process Approach. Addict Behav. 36:789-91.
57. Farber HJ, Groner J, Walley S, Nelson K. (2015) Section on Tobacco Control. Protecting Children from Tobacco, Nicotine, and Tobacco Smoke. Pediatrics. 136:1439-1467.
58. http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0102-311X2016001102001&lng=en&tlng=en
59. Record RA, Greiner LH, Wipfli H, Pugel J, Matt GE. (2022) Thirdhand Smoke Knowledge, Attitudes, and Behavior: Development of Reliable and Valid Self-report Measures. Nicotine Tob Res. 24:141-5.
60. McCaul KD, Hockemeyer JR, Johnson RJ, Zetocha K, Quinlan K, et al. (2006) Motivation to quit using cigarettes: A review. Addict Behav. 31:42-56.
61. Record RA, Greiner LH, Wipfli H, Strickland J, Owens J, et al. (2023) Evaluation of a Social Media Campaign Designed to Increase Awareness of Thirdhand Smoke among California Adults. Health Commun. 38:437-46.
62. Ferrante G, Simoni M, Cibella F, Ferrara F, Liotta G, et al. (2013) Third-hand smoke exposure and health hazards in children. Monaldi Arch Chest Dis Arch Monaldi Mal Torace. 79(1):38-43.
63. Drehmer JE, Walters BH, Nabi-Burza E, Winickoff JP. (2017) Guidance for the Clinical Management of Thirdhand Smoke Exposure in the Child Health Care Setting. J Clin Outcomes Manag. 24:551-9.
64. Matt GE, Quintana PJE, Hoh E, Zakarian JM, Dodder NG, et al. (2020) Persistent tobacco smoke residue in multiunit housing: Legacy of permissive indoor smoking policies and challenges in the implementation of smoking bans. Prev Med Rep. 18:101088.
65. Becquemin MH, Bertholon JF, Bentayeb M, Attoui M, Ledur D, et al. (2010) Third-hand smoking: indoor measurements of concentration and sizes of cigarette smoke particles after resuspension. Tob Control. 19(4):347-8.
66. Matt GE, Quintana PJE, Destaillats H, Gundel LA, Sleiman M, et al. (2011) Thirdhand tobacco smoke: emerging evidence and arguments for a multidisciplinary research agenda. Environ Health Perspect. 119(9):1218-26.