Michael Pietrangelo DO1* and Jeremy Hess DO2
1Department of Internal Medicine, New Hanover Regional Medical Center, Wilmington, North Carolina, USA
*Corresponding author: Michael Pietrangelo, Department of Internal Medicine, New Hanover Regional Medical Center, Wilmington, North Carolina, USA.
Citation: Do Pietrangelo M, DO Hess J. (2024) The double-edge of treatment: A case of double positive Anti-glomerular basement membrane disease (Goodpasture’s Syndrome). Genesis J Surg Med. 3(1):1-6.
Received: February 16, 2024 | Published: March 28, 2024
Copyright© 2024 genesis pub by DO Pietrangelo M, et al. CC-BY-NC-ND 4.0 DEED. This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-No Derivatives 4.0 International License. This allows others distribute, remix, tweak, and build upon the work, even commercially, as long as they credit the authors for the original creation.
DOI: https://doi.org/10.52793/GJSM.2024.3(1)-23
Abstract
Pulmonary-Renal Syndrome is a rare, but life-threatening syndrome characterized by necrotizing glomerulonephritis and diffuse alveolar hemorrhage (DAH), including anti-glomerular basement membrane (anti-GBM) disease. This clinical syndrome can have significant morbidity including end-stage renal disease (ESRD), chronic lung disease or even death. In this case, a previously healthy patient who was anuric for five days presented with uremia and worsening dyspnea after developing sinus congestion, shortness of breath and a dry cough. Laboratory investigation showed azotemia, hyperkalemia, and elevated creatinine. A chest x-ray was suspicious for DAH in the setting of Pulmonary-Renal Syndrome. The patient was emergently started on continuous renal replacement therapy (CRRT) and plasmapheresis was coordinated with the American Red Cross. The patient required endotracheal intubation due to worsening acidosis and hypoxia. Bronchoscopy confirmed DAH. Immunological markers prior to the initiation of plasmapheresis revealed an elevated anti-GBM antibodies, confirming the diagnosis of Goodpasture’s Syndrome. The patient’s clinical course was complicated, but was ultimately responsive to multiple courses of plasmapheresis, high dose corticosteroids and cyclophosphamide (although elevated anti-GBM titers persisted). This case demonstrates several uncommon clinically phenotypic findings of this rare disease: including double positive serology, rapid onset, severity, and DAH. Additionally, this case highlights some of the complications of treatment of Pulmonary-Renal Syndrome and the management of refractory disease. It is critical that Goodpasture’s Syndrome be considered strongly as early diagnosis leads to expedited treatment and improved outcomes in double positive disease. Unfortunately, the only definitive therapy of disease associated ESRD remains renal transplant after achievement of remission.
Keywords
Pulmonary renal syndrome; Good pasture disease; Anti-GBM; Case report
Introduction
Pulmonary-Renal Syndrome, Goodpasture Disease, anti-GBM, case report pulmonary-Renal Syndrome is due to rapidly progressive glomerulonephritis causing acute renal failure combined with diffuse alveolar hemorrhage (DAH) causing respiratory failure [1]. Generally, this disease has an insidious onset, with nonspecific symptoms preceding renal and pulmonary manifestations by weeks to months [2]. It is most commonly caused by antineutrophil cytoplasm antibody-associated vasculitis (AAV) and less commonly anti-GBM disease [3]. The anti-GBM etiology is an overall rare condition with an incidence of approximately one per million persons [4,5]. Renal prognosis, although poor overall, is more favorable in patients who have a combination of anti-GBM and c-ANCA as these patients’ renal may clinically resemble AAV more rather than anti-GBM mono-seropositive disease [6]. We present a clinical case of Pulmonary-Renal Syndrome caused by anti-GBM disease requiring admission to the intensive care unit, plasmapheresis, immunosuppressive therapy and corticosteroids. This case presents multiple complications that can be associated with Pulmonary-Renal Syndrome and its subsequent treatment. We present the following article in accordance with the CARE reporting checklist.
Case Presentation
The patient is a 77-year-old female with a past medical history of hypothyroidism and hyperlipidemia who presented initially to her primary care provider with a complaint of dyspnea for five days. She was started on antibiotics and steroids however her symptoms progressed, leading to a presentation to the emergency department two days later. On arrival, she provided an additional history of associated sinus congestion, anorexia, nausea, vomiting, and anuria. Initial vitals reflected hypoxia and laboratory results significant for hyperkalemia of 6.4 mmol/L, a serum bicarbonate 10.0 mmol/L with an anion gap of 25, a creatinine of 14.11 mg/dL, BUN 212 mg/dL, phosphorous 11.5 mg/dL, lactic acid 1.0 mmol/L, C-reactive protein 20.5 mg/dL, white blood cell 23.1 K/μL and hemoglobin 6.8 g/dL requiring transfusion. Initial arterial blood gas (ABG) showed pH 7.37, pCO2 20.3 mmHg, pO2 75 mmHg indicating a metabolic acidosis with respiratory alkalosis. Initial chest X-ray showed bilateral lung consolidations as seen in (Figure 1) consistent with diffuse alveolar hemorrhage. Renal ultrasound showed no evidence of urinary obstruction. Nephrology was consulted and the patient consented to emergency continuous renal replacement therapy (CRRT). She required supplemental oxygen via nasal cannula at first but later needed high flow oxygen. A repeat ABG showed worsening hypercapnic acidosis. Due to increasing oxygen requirements, uremic encephalopathy and the need for a bronchoscopic evaluation, the patient was electively incubated and transferred to the intensive care unit (ICU).

Figure 1: Original image of initial chest X-ray post-intubation with bilateral pulmonary opacities seen in diffuse alveolar hemorrhage.
In the ICU, bronchoscopy demonstrated bright red foam covering the mucosa, with oozing blood in all lobes, supporting DAH. Bronchoalveolar lavage (BAL) yielded respiratory epithelial cells with scattered macrophages and moderate acute inflammation and no evidence of infections. Pulse dose steroids were started with methylprednisolone and hemodialysis access was obtained and CRRT was initiated. The patient became hypotensive requiring packed red blood cells and a norepinephrine infusion was initiated. Continuous bicarbonate infusion was added due to severe acidosis. Also, with leukocytosis with hypotension, empiric antibiotics were initiated due to suspicion for community acquired pneumonia. Autoimmune workup revealed no antinuclear antibodies (ANA), no perinuclear anti-neutrophil cytoplasmic antibodies (p-ANCA), no cryoglobulins or rheumatoid factor. She tested positive for anti-neutrophil cytoplasmic antibodies (c-ANCA) at 1:40 and her anti-GBM antibodies were markedly elevated at 205 units. Plasmapheresis was coordinated with the American Red Cross, initially every day, but was transitioned to every other day after the first 4 rounds due to concerns for removal of clotting factors. CRRT was transitioned to three times weekly hemodialysis, which the patient tolerated well.
While the patient was receiving plasmapheresis, she was also treated with oral cyclophosphamide and prednisone. With the appearance of a severe rash, cyclophosphamide was replaced with mycophenolate mofetil and there was a subsequent increase in her anti-GBM antibodies levels. Mycophenolate mofetil was discontinued and the patient was rechallenged with a lower dose of intravenous cyclophosphamide which she tolerated well.
After the initial round of plasmapheresis completed, the patient was weaned off mechanical ventilation. However, the patient developed worsening pulmonary edema and hypoxia, requiring reintubation. She was volume challenged and had another 10-session course of plasmapheresis due to concerns for relapsing disease. Consequently, her anti-GBM antibodies decreased and then subsequently plateaued (Figure 2). A renal biopsy was considered for prognostication and duration of immune suppression, but held due to the patient’s unstable respiratory status.
Figure 2: Graph of serum anti-GBM levels with dates of plasmapheresis procedures over time.
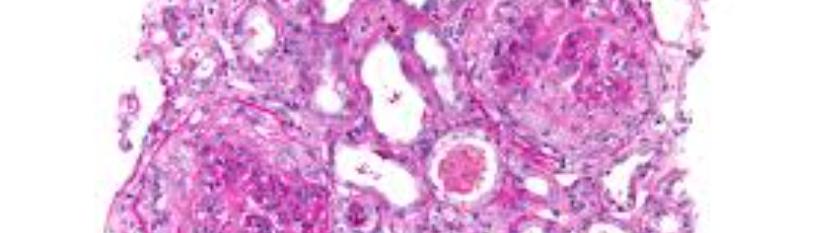
Prior to the completion of her tenth plasmapheresis session, the patient developed acute worsening shortness of breath and a stat chest X-ray showed pneumoperitoneum from a viscus perforation. She was intubated and underwent emergent exploratory laparotomy for closure of a steroid associated perforated stress duodenal ulcer with modified Graham patch repair and Jackson-Pratt drain placement. Because of these complications and the potential for hemorrhage, further plasmapheresis was held unless the anti-GBM antibodies rapidly increased. Rituximab was considered, but ultimately held as her anti-GBM titers did not persist past six weeks. Again, she was weaned off of mechanical ventilation to low flow nasal cannula oxygen. Over the next two months, she clinically improved, save for continued dialysis dependence. She underwent a renal biopsy (Figure 3) and was discharged to rehab in good condition. Her biopsy results showed late stage anti-GBM glomerulonephritis, specifically, with all glomeruli globally sclerotic; some with fibrous crescents. Light microscopy was pertinent for moderate tubular atrophy and interstitial fibrosis and moderate-to-severe arterial thickening without vascular inflammation or necrosis. Immunofluorescence demonstrated polyclonal IgG positive linear staining, a classic finding in Anti-GBM disease (Figure 4). At her post-hospital follow up, the patient was stable, tolerating hemodialysis well. Thereafter, she was weaned off of immunosuppression. Her renal function was declared as end-stage and she transitioned to peritoneal dialysis. The patient is currently pursuing a renal transplant via a living donor.

Figure 3: Original renal ultrasound image with biopsy needle.
Figure 4: Original pathology slides showing: A) Medium-power paraffin Hematoxylin and Eosin (H&E) staining – All of the glomeruli are globally or almost globally sclerotic, some with associated fibrous crescents. B) Medium-power frozen-section H&E – all glomeruli globally sclerotic. C) IgG immune fluorescence - Glomerular capillaries exhibit diffuse, linear staining.
Discussion
In patients who present with acute renal failure with suspected pulmonary infiltrates on chest imaging, Pulmonary-Renal Syndrome must be considered and excluded. Pulmonary- Renal Syndrome is characterized by the combination of rapidly progressive glomerulonephritis and pulmonary capillaritis, which is diagnosed on histological analysis with a prevalence of 1 in 1,000,000 people. This is commonly caused by necrotizing small vessel vasculitis, anti-GBM disease, antiphospholipid syndrome, hypertensive nephrosclerosis, hemolytic uremic syndrome, or endocarditic [1]. Specifically, anti-GBM disease is caused by circulating auto-antibodies against the carboxyl terminal non-collagenous (NC1) domain of the alpha-3 chain of type IV collagen, which is one of six alpha-chains found in basement membrane collagen. Although the specific antigenic stimulus is not well understood, many cases are precipitated by prior renal or pulmonary injury, infection or environmental exposure [6]. Immunologic dysregulation and genetic susceptibility have also been implicated as potential contributing factors [6]. About 32% of cases of anti-GBM disease patients are co-seropositive for ANCA antibodies, known as “double-positive” [7]. Double-positive antibodies are antigenically distinct, not due to cross reactivity and the pathogenesis of these antibodies are not well understood. Although presenting with phenotypic dominance of anti-GBM disease, prognostically double positive disease resembles AAV; with similar renal susceptibility to treatment and increased relapse rate, requiring longer maintenance immune suppression [8]. c-ANCA positivity is specific for systemic vasculitis [9]. c-ANCA and associated damage of the basement membrane is hypothesized to reveal an epitope which normally is hidden from circulating anti-GBM antibodies.
The initial workup when suspecting Pulmonary-Renal Syndrome includes checking the anti-GBM titers, c-ANCA, p-ANCA, complement C3 and C4. If ANCA is positive, it points towards Wegener’s granulomatosis, microscopic polyangiitis or Churg–Strauss syndrome and can be further differentiated by indirect immunofluorescent staining for cytoplasmic ANCA (c-ANCA) vs perinuclear (p-ANCA). The target for c-ANCA is proteins 3 (Pr3) and p-ANCA is myeloperoxidase (MPO). The importance of trending the anti-GBM titers is essential as this is known to approximately correlate with disease severity [1]. In 80% of cases, HLA alleles DR4 or DR15 are present, while DR1 and DR7 are significantly less common [4]. Anti-GBM antibodies have a sensitivity of 95–100% and a specificity of 90–100% when used to diagnose Goodpasture’s syndrome [13]. Bronchoscopy is essential to confirm DAH, an indication for treatment including plasmapheresis.
Treatment for Pulmonary-Renal Syndrome is multi-pronged and focused on preventing further damage as well as reversing the autoimmune effects on the body. As seen in this case, management is divided into the acute phase and the maintenance phase. It is not uncommon to see an infectious workup performed with cultures and antibiotics, especially if symptoms mimic that of pneumonia with fevers and cough. Initial treatment involves immune suppression with high dose steroids and a generally accepted regimen is methylprednisolone 1000 mg for three to five days followed by a long taper. Cyclophosphamide is the cytotoxic treatment of choice but mycophenolate mofetil or Rituximab can be substituted. Initiation of plasmapheresis and immunosuppression are indicated when a patient presents with DAH or does not require dialysis on admission. However, if the patient requires dialysis or does not have DAH, treatment is controversial. Due to this patient’s hyper-acute presentation, DAH and double seropositivity plasmapheresis was initiated. Maintenance phase is achieved in approximately 85% of patients treated and generally begins 6-12 months after the acute phase and low dose immunosuppression is used as well as hemodialysis if renal failure persists [14]. In patients who progress to ESRD a renal transplant is indicated if they convert to zero negativity, due to risk of recurrent disease.
References
1. Sanders JS, Rutgers A, Stegeman CA, Kallenberg G M C. (2011) Pulmonary-renal syndrome with a focus on anti-GBM disease. Semin Respir Crit Care Med. 32(3):328-34.
2. Kluth DC, Rees AJ. (1999) Anti-glomerular basement membrane disease. J Am Soc Nephrol. 10(11):2446.
3. West S, Arulkumaran N, Ind PW, Pusey CD. (2013) Pulmonary-renal syndrome: a life threatening but treatable condition. Postgrad Med J. 89(1051):274-83.
4. Papiris SA, Manali, ED, Kalomenidis I, Kapotsis GE, Karakatsani A, et al. (2007) Bench-to-bedside review: Pulmonary–renal syndromes – an update for the intensivist”. Crit Care 11(3):213.
5. McAdoo SP, Pusey CD. (2017) Anti-Glomerular Basement Membrane Disease.” Clin J Am Soc Nephrol. 12(7):1162-72.
6. https://search.worldcat.org/title/comprehensive-clinical-nephrology/oclc/698079097
7. Levy JB, Hammad T, Coulhart A, Dougan T, Pusey CD. (2004) Clinical features and outcome of patients with both ANCA and anti-GBM antibodies. Kidney Int. 66(4):1535–40.
8. McAdoo SP, Tanna A, Hruskova Z, Holm L, Maria Weiner, et al. (2017) Patients double-seropositive for ANCA and anti-GBM antibodies have varied renal survival, frequency of relapse, and outcomes compared to single-seropositive patients. Kidney Int. 92(3):693-702.
9. Jayne DR, Marshall PD, Jones SJ, Lockwood CM. (1990) Autoantibodies to GBM and neutrophil cytoplasm in rapidly progressive glomerulonephritis. Kidney Int. 37(3):965-70.
10. Serratrice J, Chiche L, Dussol B, Granel B, Daniel L, et al. (2004) Sequential development of perinuclear ANCA-associated vasculitis and anti-glomerular basement membrane glomerulonephritis. Am J Kidney Dis. 43(3):e26-e30.
11. Bolton WK. (1996) Goodpasture's syndrome. Kidney Int. 50(19):1753-66.
12. Kelly PT, Haponik EF. (1994) Goodpasture Syndrome: Molecular and Clinical Advances”. Medicine. 73(4):171-185
13. Salama A, Dougan T, Levy J, Cook HT, Morgan SH, et al. (2002) Goodpasture’s disease in the absence of circulating anti-glomerular membrane antibodies as detected by standard techniques. Am J Kidney Dis. 39(6):1162-67.
14. Hogan S, Falk RJ, Chin H, Cai J, Jennette CE, et al. (2005) Predictors of relapse and treatment resistance in antineutrophil cytoplasmic antibody-associated small vessel vasculitis. Ann Intern Med. 143(9):621-31.