Soft Tissue Augmentation of Dental Implants -Before, During and After Implant Placement
Sheng Zhang* and Kenneth Lee2
1BDS (Adel), Grad Dip Ortho (JCU), FRACDS; Private practice, Dental Artistry, 38 Broadway Newmarket, Auckland, 1023 New Zealand
2Professor Universitat Jaume I, Castellon, BDS (Syd), MSc Oral Implantology (Goethe), MSc Orthodontics (Castellon), FICD, FPFA, Private practice, Sydney, Australia
*Corresponding author: Sheng Zhang, Private Practice, Dental Artistry, 38 Broadway Newmarket, Auckland, 1023 New Zealand.
Citation: Zhang S, Lee K. (2022) Soft Tissue Augmentation of Dental Implants-Before, During and After Implant Placement. J Oral Med and Dent Res. 3(1):1-13.
Received: March 09, 2022 | Published: May 13, 2022
Copyright© 2022 Genesis Pub by Zhang S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC BY 4.0). This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author(s) and source are properly credited.
DOI: https://doi.org/10.52793/JOMDR.2020.3(1)-24
Abstract
Background: Dental implants have traditionally been focused on bone quality and quantity to house the dental implant for successful restoration of the edentulous ridge. Rough surface implants provide predictable osseointegration but results in the development of peri-implantitis when exposed to the oral environment. The peri-implant mucosa provides protection to the underlying bone via its immune response and protection from apical biofilm migration. An adequate band of attached keratinized mucosa also improves comfort with performing oral hygiene, limit early marginal bone loss and improved aesthetic outcomes around implant prostheses.
Aim: The purpose of this article is to provide a literature review on the importance of attached keratinized mucosa around dental implants. It also shares simple strategies to improve the peri-implant mucosa before, during and after implant placement.
Discussion: There is a paradigm shift in dental implantology with an increasing importance placed on the quality and quantity of soft tissue around dental implants to improve the surgical and prosthetic outcomes of implant therapy.
Keywords
Bone remodeling; Dental implants; Implantology; Implant mucosa augmentation; Peri-implant mucosa; Soft tissue complications
Introduction
Since Branemark first discovered osseointegration in 1962 and subsequently went on to place the first dental implant in a human patient the field of dental implantology was born [1]. Over the next half century significant advances have resulted in more predictable treatment results with shortened treatment durations. The first machined surface implants had a healing period of 3-6 months. The introduction of roughened surfaces has shortened the healing period to 3-4 months with more osteogenic surface treatments advocating 6-8 weeks and biologically active hydrophilic surfaces reducing this further to just 3-4 weeks [2].
This trend demonstrates a clear understanding of the osseointegration process, and it is estimated 12-18 million implants are placed annually around the world with high survival rates (Bjorn 2018). With the advent of rough surface implants and their wide spread use the prevalence of peri-implantitis increased with the phenomenon first reported in 1987 around rough surface titanium plasma sprayed implants [3].
Soft Tissue Around Dental Implants
Peri-implantitis is a plaque-associated pathological condition occurring in tissues around dental implants, characterized by inflammation in the peri-implant mucosa and subsequent progressive loss of supporting tissue [4]. As such it must be emphasized that the inflammatory process is in the soft tissue and bone resorption occurs as a direct result of this inflammation. The prevalence of peri-implantitis ranges from 9.6%-22% at the implant level [5-7].
Peri-implantitis must be differentiated from physiological bone remodeling [8]. Although both result in the loss of bone around the implant their aetiology is different. Early marginal bone loss is a non-infective physiological process, occurring within the first year after implant placement [9]. The aetiology is complex and includes surgical and prosthetic factors such as bone overheating, implant placement torque and depth, the number of abutment disconnections and the implant abutment microgap. Early marginal bone loss is hence an adaptive response of the peri-implant tissues to these factors. Early marginal bone loss greater than 0.44mm in the first 6 months after prosthetic loading serves as a risk indicator for peri-implant bone loss progression at 18 months [10]. Progression of crestal bone loss is rare around dental implant in the absence of inflammation therefore early bone remodeling may initiate peri-implantitis [4].
Berglundh and Lindhe showed the peri-implant mucosa re-establishes itself as a “Biological Width” (now termed supracrestal tissue height)around implants [11]. In the presence of thin mucosa, it was found bone resorption took place around the implant creating an angular defect to allow an increase in soft tissue thickness. It was thus concluded a minimum thickness of soft tissue was required around implants to allow for healing. This minimum thickness is 3-4mm in humans with an epithelial coronal component that varies between 2.0 – 2.2mm and an apical zone of connective tissue adhesion between 1.1-1.7mm wide [12,13]. The soft tissue vertical dimension around implants on average is 1-1.5mm more than around teeth [14]. The epithelial component share similarities between the implant and tooth however the connective tissue shows histological differences. The orientation of the collagen fibers with the connective tissue runs parallel with the abutment body compared to natural teeth whereby fibers attach into the cementum at perpendicular and oblique angles [15]. Supracrestal tissue height is independent of implant design and restorative modality [16].
The increased prevalence of peri-implant bone loss and the more rapid progression of periodontal disease around implants compared to teeth [17] has resulted in a paradigm shift with a focus on the importance of the peri-implant soft tissue in its ability to protect the underling bone and limit early marginal bone loss. Albrektsson [18] recently coined this a new era of mucointegration.
Avila-Ortiz’s group recently proposed a new term the ‘peri-implant phenotype’. The term encompasses the soft tissue component comprised of peri-implant keratinised mucosa width, mucosa thickness and supracrestal tissue height as well as the hard tissue compartment comprising the peri-implant bone thickness [16].
The significance of keratinised mucosa has been controversial around teeth and implants alike [19]. The 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions found equivocal evidence on the presence or absence of keratinized mucosa on the health of the peri-implant tissues. The consensus statement however states “keratinized mucosa may have advantages regarding patient comfort and ease of plaque removal” [8]. There is growing evidence to support the minimum amount of 2mm of keratinized mucosa width to minimize the development of peri-implant disease especially in patients with poor maintenance compliance [20].
The peri-implant mucosa can be measured in thickness and height. Together they give volume to the tissue. Thickness relates to the horizontal dimension of the peri-implant soft tissue and height refers to the supracrestal tissue height akin to the supracrestal tissue attachment on teeth. The vertical dimension is significant due to its effect on early bone remodeling as discussed previously.
Mucosa thickness may contribute to the health of the implant with better maintenance of marginal bone heights [21]. Most of the research investigating mucosal thickness examine its effect on aesthetics of the implant by its ability to mask the colour of the underling restorative abutment. Tissue of 1.5mm thickness or less resulted in visible tissue colour changes however, 2mm thick tissue was able to mask zirconia substrates and 3mm tissue thickness was able to mask all restorative materials [22].
Increased peri-implant mucosa results in more volume of extracellular matrix and collagen but also increase vascularity which improves immune response, enhanced clearance of toxic and growth factor migration [23]. This immune response has been suggested to be more important than the tissue attachment to the implant to prevent apical biofilm migration [12]. Thicker tissue also responds favorably to flap management, wound healing, restorative trauma and less post prosthetic recession [24].
Mucosal thickness varies in the mouth with the maxilla generally thicker than the mandible. The mandibular edentulous molar ridge exhibits the thinnest soft tissue thickness and this area often poses the greatest challenge for the clinician [25].
When examining a site for dental implant placement, the current paradigm shift dictates that we must evaluate not just the availability of bone to house the implant but also the quality of the soft tissue at the site including the width of keratinized mucosa and the thickness and height of the mucosa.
Soft Tissue Augmentation Around Dental Implants
Soft tissue augmentations at sites lacking the required soft tissue can take place prior to implant placement, during implant placement, at second stage implant uncover surgery or post prosthetic loading. There does not appear to be a difference between staged or simultaneous soft tissue augmentation during implant placement and procedures can significantly enhance keratinized tissue width and mucosal tissue thickness with stability of tissues obtained 3 months post augmentation [26].
Traditional mucogingival surgeries such as frenectomies, free gingival grafts, apical repositioning flaps and alveolar ridge augmentations are applicable to the peri-implant soft tissues. Autologous soft tissue grafts still provide the gold standard in gains of keratinized tissue and mucosal thickness compared to autologous substitutes on the market [21]. Soft tissue complications around dental implants in most cases fall into the category of volume deficiency, lack of attached mucosa and recession around implants.
This article shall provide a summary of common augmentation procedures available to the implant clinician at different stages of implant therapy to address the above three soft tissue complications. It will assume the use of autologous soft tissue grafts however autologous substitutes can be readily substituted in many of the illustrated procedures [27]. The efficacy of these substitutes is not within the scope of this article.
Soft Tissue Procedures Prior to Implant Placement
As a part of a comprehensive implant restorative work up the current soft tissue status must be documented. This may be an edentulous ridge or dentate ridge with the compromised tooth still in situ. The assessment involves determination of any mucogingival defects such as frenulum, amount of attached mucosa and volume of soft tissue. Different methods of determining soft tissue phenotype have been suggested with visual inspection, radiographic assessment, using probes and calipers [28,29]. Having a clear understanding of the soft condition prior to implant placement allows for adoption of strategies to mitigate the risks associated with them.
Frenectomy may be performed prior to implant placement to improve the surgical outcomes. High frenum attachments can increase flap mobility and is a major cause of surgical wound opening. Apical positioned flaps (APF) (Figure 1 A-B) can be performed to increase the band of attached keratinized tissue and increase the depth of the vestibule to facilitate oral hygiene and allow periosteal releasing incisions and flap advancement at later surgeries.
The APF technique has several advantages: It does not require a second surgical site, results in minimal postoperative bone loss, controls postoperative gingival margin status, and has higher patient acceptance (Martins et al., 2010). When the soft tissue phenotype requires modification an APF with a free gingival graft can prepare the site for ideal implant placement at a later stage (Figure 1C).
Figure 1: (A) Submarginal incision for an APF; (B) APF sutured to the periosteum; (C) APF with free gingival graft with periosteal sling sutures.
When an extraction and socket preservation is indicated for a delayed implant placement at a site with thin phenotype or loss of attached keratinized tissue then a socket sealing procedure is recommended to improve and modify the soft tissue at the extraction site. A biopsy punch can be used to harvest a free gingival graft from the palate or maxillary tuberosity to be transferred to the extraction socket with or without bone graft substitute (Figure 2A-C). In cases where additional volume gain in soft tissue is desirable at the labial or lingual aspects then a saddle socket seal provides the most soft tissue volume gain of all the socket preservation techniques [30,31] (Figure 2D-E).
Figure 2: (A) Tissue biopsy punch is used to harvest a round free gingival graft from the palate or tuberosity; (B) Donor site after free gingival graft harvest; (C) Socket seal occlusion of extraction socket using free gingival graft secured with figure of 8 suture; (D) Rectangular free gingival graft partially de-epithelised to be used as a “Saddle” socket seal; (E) Saddle socket seal de-epithelised graft is tunneled under the buccal and palatal gingiva and secured with simple interrupted sutures.
In the anterior maxilla, extraction of a tooth in the presence of a thin buccal bone plate (<1mm) can result in the spontaneous thickening of the soft tissue by 7 fold after an 8 week healing period [32] (Figure 3C). With appropriate case selection which exhibit favorable sagittal root position and palatal and apical bone volume, this phenomenon affords the patient a “free” connective tissue graft when a Type II early implant placement protocol is adopted. In these cases, no socket preservation is required at the time of tooth extraction and Type 2 placement with simultaneous buccal bone contour augmentation protocol has been shown to yield stable long term functional and cosmetic results [33].
Figure 3: (A) & (B) 6 weeks after extraction of upper left lateral incisor without socket preservation the ridge appears to have maintained the original soft and hard tissue architecture; (C) Elevation of a papilla sparing flap reveals the ridge is maintained by a spontaneous thickening of soft tissue and there is complete loss of the buccal plate.
Appropriate assessment of soft tissue prior to implant is essential to allow for correction of the deficiencies identified. Managing the soft tissue prior to implant placement prevents or reduces the risks of future complications which may be more difficult to manage once the implant has been placed into the site.
Soft Tissue Procedures During Implant Placement
Implant specific strategies are available to supplement vertical soft tissue deficiencies. These include flattening of the ridge, subcrestal placement, soft tissue tenting and soft tissue mucogingival augmentation [34]. The advantage of soft tissue augmentation at the time of implant placement include shorter overall treatment times; fever surgeries; enables simultaneous hard tissue and soft tissue healing; results in a shorter healing time; produces less pain and discomfort; causes less stress; lowers the costs; and provides greater patient satisfaction [35]. The risks arise from combining multiple surgical procedures at one site which may compromise vascularity and increases the likelihood of post operative complications. The surgery also becomes technically more challenging. Soft tissue augmentation can accompany implant surgery as one stage or stage procedure.
Free gingival grafts and subepithelial connective grafts have been proposed as effective in increasing soft tissue volume around implants [36]. Immediate Type 1 implant placements is associated with soft tissue recession and buccal plate loss regardless of surgical approach [37]. Even with the use of bone graft material in the jump gap the ridge width often diminishes [38]. Simultaneous connective tissue grafts at the time of implant placement in the aesthetic zone can counteract these dimension changes and maintain the ridge architecture regardless of the soft tissue phenotype at the site [39] (Figure 4A-C). These changes can be maintained long term and provide a better aesthetic result for the patient reducing the risk of recession [40,41].
As a two-stage implant surgery soft tissue grafts such as CTG can be placed and primary surgical closure can be achieved. This provides an excellent environment for graft revascularization and allows the operator a ‘second’ opportunity to further improve the soft tissue if required at the second stage implant surgery (Figure 4D-F). Soft tissue augmentation procedures for one stage implant placement are similar for strategies to gain soft tissue volume at the implant uncover stage which is described in the next section.
Figure 4: (A) Upper left central incisor crown fractured subgingival; (B) Upper left central incisor root has been extracted and a connective tissue graft has been harvested from the palate ready to place under the buccal gingiva; (C) Connective tissue graft has been secured under the buccal gingiva and immediate implant placed with healing abutment; (D) Implant placement at the lower right first molar site with cover screw; (E) An allograft soft tissue matrix (Alloderm RTM) has been used as an autologous soft tissue substitute; (F) The site is primary closed with the allograft soft tissue matrix buried.
Soft Tissue Procedures after Implant Placement
Implants which are placed with a two stage approach offers opportunities to further improve the soft tissue at the implant second stage surgery. There are many procedures proposed by authors, each with their indications and desired outcomes. These procedures can be adopted for one stage implant placements. Where possible split thickness flaps are recommended to limit the amount of bone resorption around the implants associated with lifting the periosteum [42].
The strategies to gain attached mucosa and tissue volume defers in the maxilla and the mandible. The maxilla is fortunate to have a large supply of attached keratinized tissue on the palate which can be readily mobilized during second stage surgery (Figure 5A-F) where the mandible may need a free graft to modify the tissue quality (Figure 5G-I).
Figure 5: (A) An implant ready to uncover in the maxilla; (B) De-epithelising the palatal keratinised attached mucosa; (C) Split thickness flap elevation from the palate; (D) Split thickness flap extended to the buccal aspect; (E) Palatal aspect of the flap is folded on itself and secured with a horizontal mattress suture; (F) The roll flap is approximated next to the healing abutment; (G) & (H) Uncover of an implant in the mandible where there is limited tissue volume to mobilise from the lingual aspect. An APPTF is prepared and secured to the periosteum; (I) A free gingival graf is adapated around the implant healing abutment to enhance the tissue at the site.
To gain keratinized attached mucosa in the maxilla an apically positioned partial thickness flap (APPTF) is recommended. In the mandible AAPTF with free gingival graft in the lower jaw [43]. To gain volume a roll envelope flap is recommended in the maxilla and APPTF with a connective tissue graft is recommended in the mandible.
In the aesthetic zone there is the added challenge of creating tissue volume which recreates papilla form around the implant prosthesis. This aids in not just aesthetics but also removes potential food traps at the gingival embrasure. Two easily adopted techniques at second stage surgery are the split finger technique [44] (Figure 6A-F) and the Palacci flap [45] (Figure 7A-G).
Figure 6: Split finger technique to create papillary soft tissue volume and gain of vertical tissue; (A),(B),(C) Schematic of the incision to be made in the crest to create the surgical papillas; (D) The surgical papillas are elevated and approximated buccal to palatal to form the new anatomic papilla; (E) & (F) Vertical mattress sutures are used to evert and secure the surgical papillas together creating volume and height.
Figure 7: Palacci Flap for two adjacent implants; (A) & (B) Schematic for the incisions for Palacci flap showing the semilunar bevel incisions on the crest; (C) Flap is raised and healing abutments attached to the implants; (D) The semilunar bevels are pedicle flaps which can be rotated between the implants and the adjacent teeth; (E) Flap is sutured to hold the pedicles in place; (F) An edentulous ridge with two implants ready for uncover. Palacci flaps were raised and healing at 3 weeks shows excellent soft tissue volume gain on the buccal and between the implants.
Soft tissue augmentation around osseointegrated and restored implants are also possible. This is commonly performed to correct a recession defect which is an aesthetic or plaque retention concern. In these cases, a coronally advanced flap with a connective tissue graft yields the best result [46] (Figure 8 A-D). The use of connective tissue grafts sourced from the maxillary tuberosity tended to give better long-term stability and increase keratinized tissue gain compared to graft harvested from the lateral palate [47-49].
Figure 8: Schematic of a coronally advanced connective tissue graft around an implant with healing abutment; (A) Full thickness flap is raised on the buccal aspect requiring augmentation; (B) A connective tissue graft is shaped and approximated to the recipient site; (C) The connective tissue graft is secured to stable tissue with simple interrupted sutures; (D) The flap is primary closed over the connective tissue graft.
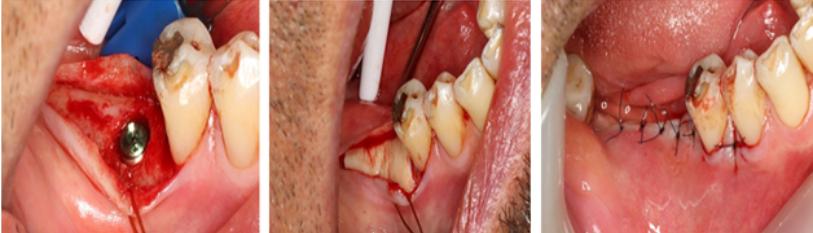
Figure 9: (A) & (B) Schematics of a submarginal free gingival graft with APF around a restored dental implant. A submarginal approach reduces the risks the procedure worsening soft tissue recession around the implant; (C) A case with implants in the lower right quadrant replacing teeth 35,36 and 37. The site has no attached keratinized mucosa and if tender to oral hygiene and bleeds on proving; (D) Submarginal AAPTF; (E) FGG sutured to the periosteum bed; (F) 3-month review of the site showing an excellent band of attached keratinized mucosa. There is no more discomfort in performing oral hygiene and the site has healthy periodontal attached tissue with no bleeding on probing.
Conclusion
The increased awareness of the importance of the quantity and quality of the peri-implant soft tissue has resulted in a paradigm shift from a bone driven implant planning process to one that places equal importance to the soft tissue. As Jan Lindhe famously said, “the bone sets the tone but the soft tissue is the issue”. Soft tissue deficiencies can be predictable managed at multiple time points in the implant planning and surgical phases. Early management is recommended to improve the surgical and prosthetic outcomes of implant therapy.
Acknowledgments
The author thanks Dr Annie Dou for her contributions to the drawings.
References
- Albrektsson T, Chrcanovic B, Jacobsson M, Wennerberg A. (2017) Osseointegration of implants: a biological and clinical overview. JSM Dent Surg. 2(3):1-6.
- Buser D, Broggini N, Wieland M, Schenk RK, Denzer AJ, et al. (2004) Enhanced Bone Apposition to a Chemically Modified SLA Titanium Surface. J Dent Res. 3(7):529-33.
- Mombelli A, van Oosten MA, Schurch E, Jr, Land NP. (1987) The microbiota associated with successful or failing osseointegrated titanium implants. Oral Microbiol Immunol. 2(4):145-51.
- Schwarz F, Derks J, Monje A, Wang HL. (2018) Peri-implantitis. J Clin Periodontol. 20:246-66.
- Atieh MA, Alsabeeha NH, Faggion CM, Duncan WJ. (2013) The frequency of peri-implant diseases: a systematic review and meta-analysis. J Periodontol. 84(11):1586-98.
- Derks J, Tomasi C. (2015) Peri-implant health and disease. A systematic review of current epidemiology. J Clin Periodontol. 16:158-71.
- Rakic M, Galindo-Moreno P, Monje A, Randovanovic S, Wang HL, et al. (2018) How frequent does peri-implantitis occur? A systematic review and meta-analysis. Clin Oral Investig. 22(4):1805-16.
- Berglundh T, Armitage G, Araujo MG, Avila-Ortiz G, Blanco J, et al. (2018) Peri-implant diseases and conditions: Consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Periodontol. 1:313-18.
- Lombardi T, Berton F, Salgarello S, Barbalonga E, Rapani A, et al. (2019) Factors Influencing Early Marginal Bone Loss around Dental Implants Positioned Subcrestally: A Multicenter Prospective Clinical Study. Journal of Clinical Medicine. 8(8):1168.
- Galindo-Moreno P, León-Cano A, Ortega-Oller I, Monje A, Valle FO, et al. (2015) Marginal bone loss as success criterion in implant dentistry: beyond 2 mm. Clin Oral Implants Res. 26(4):28-34.
- Berglundh T, Lindhe J. (1996) Dimension of the periimplant mucosa. J Clin Periodontol. 23(10):971-73.
- Tomasi C, Tessarolo F, Caola I, Piccoli F, Wennstrom JL, et al. (2016) Early healing of peri‐implant mucosa in man. J Clin Periodontol. 43(10):816-824.
- Tomasi C, Tessarolo F, Caola I, Wennström J, Nollo G, et al. (2014) Morphogenesis of peri-implant mucosa revisited: an experimental study in humans. Clin Oral Implants Res. 25(9):997-03.
- Parpaiola A, Cecchinato D, Toia M, Bressan E, Speroni S, et al. (2015) Dimensions of the healthy gingiva and peri-implant mucosa. Clin Oral Implants Res. 26(6):657-662.
- Ivanovski S, Lee R. (2018) Comparison of peri‐implant and periodontal marginal soft tissues in health and disease. Periodontol 2000. 76(1):116-130.
- Avila-Ortiz G, Gonzalez-Martin O, Couso-Queiruga E, Wang HL.(2020) The peri-implant phenotype. J Periodontol. 91(3):283-88.
- Heitz-Mayfield LJ, Lang NP. (2010) Comparative biology of chronic and aggressive periodontitis vs. peri-implantitis. Periodontol 2000. 53:167-181.
- Albrektsson T. (2019) New implant designs and improved surface chemistry. Clin Implant Dent Relat Res. 21(1):3-3.
- Lin G-H, Chan H-L, Wang H-L. (2013) The Significance of Keratinized Mucosa on Implant Health: A Systematic Review. J Periodontol. 84(12):1755-1767.
- Monje A, Blasi G. (2019) Significance of keratinized mucosa/gingiva on peri-implant and adjacent periodontal conditions in erratic maintenance compliers. J Periodontol. 90(5):445-53.
- Thoma DS, Naenni N, Figuero E, Hammerele C, Schwarz F, et al. (2018) Effects of soft tissue augmentation procedures on peri-implant health or disease: A systematic review and meta-analysis. Clin Oral Implants Res.15:32-49.
- Jung RE, Sailer I, Hämmerle CH, Attin T, Schmidlin P. (2007) In vitro color changes of soft tissues caused by restorative materials. Int J Periodontics Restorative Dent. 27(3):251-57.
- Nauta A, Gurtner G, Longaker MT. (2011) Wound healing and regenerative strategies. Oral Dis. 17(6):541-49.
- Evans CD, Chen ST. (2008) Esthetic outcomes of immediate implant placements. Clin Oral Implants Res. 19(1):73-80.
- Munakata M, Nagata K, Sanda M, Kawamata R, Sato D, et al. (2021) Variations in vertical mucosal thickness at edentulous ridge according to site and gender measured by cone-beam computed tomography. Int J Implant Dent. 7(1):34.
- Lin CY, Chen Z, Pan WL, Wang HL.(2018) Impact of timing on soft tissue augmentation during implant treatment: A systematic review and meta-analysis. Clin Oral Implants Res. 29(5):508-21.
- Thoma DS, Buranawat B, Hämmerle CH, Held U, Jung RE. (2014) Efficacy of soft tissue augmentation around dental implants and in partially edentulous areas: a systematic review. J Clin Periodontol. 15:77-91.
- Kan J, Morimoto T, Runcharassaeng K, Roe P, Smith D. (2010) Gingival Biotype Assessment in the Esthetic Zone: Visual Versus Direct Measurement. Int J Periodontics Restorative Dent. 30(3):237-43.
- Kloukos D, Koukos G, Doulis I, Sculean A, Stavropoulos A, et al. (2018) Gingival thickness assessment at the mandibular incisors with four methods: A cross-sectional study. J Periodontol. 89(11):1300-09.
- Vanhoutte V, Rompen E, Lecloux G, Rues S, Schmitter M, et al. (2014) A methodological approach to assessing alveolar ridge preservation procedures in humans: soft tissue profile. Clin Oral Implants Res. 25(3):304-9.
- Lambert F, Vincent K, Vanhoutte V, Seidel L, Lecloux G, et al. (2012) A methodological approach to assessing alveolar ridge preservation procedures in humans: hard tissue profile. J Clin Periodontol. 39(9):887-94.
- Chappuis V, Engel O, Reyes M, Shahim K, Nolte L-P, et al. (2013) Ridge Alterations Post-extraction in the Esthetic Zone:A 3D Analysis with CBCT. J Dent Res. 92:195-201.
- Jensen SS, Bosshardt DD, Gruber R, Buser D. (2014) Long-term stability of contour augmentation in the esthetic zone: histologic and histomorphometric evaluation of 12 human biopsies 14 to 80 months after augmentation. J Periodontol. 85(11):1549-56.
- Linkevicius T. (2019) Zero Bone Loss Concepts. Quintessence.
- Kadkhodazadeh M, Amid R, Kermani ME, Mirakhori M, Hosseinpour S. (2017) Timing of soft tissue management around dental implants: a suggested protocol. Gen Dent. 65(3):50-6.
- Thoma DS, Mühlemann S, Jung RE. (2014) Critical soft-tissue dimensions with dental implants and treatment concepts. Periodontol 2000. 66(1):106-18.
- Bakkali S, Rizo-Gorrita M, Romero-Ruiz M-M, Gutiérrez-Pérez JL, Torres-Lagares D, et al. (2021) Efficacy of different surgical techniques for peri-implant tissue preservation in immediate implant placement: a systematic review and meta-analysis. Clin Oral Investig. 25(4):1655-75.
- Sanz M, Lindhe J, Alcaraz J, Sanz-Sanchez I, Cecchinato D. (2017) The effect of placing a bone replacement graft in the gap at immediately placed implants: a randomized clinical trial. Clin Oral Implants Res. 28(8):902-10.
- Kan JY, Rungcharassaeng K, Morimoto T, Lozada J. (2009) Facial gingival tissue stability after connective tissue graft with single immediate tooth replacement in the esthetic zone: consecutive case report. J Oral Maxillofac Surg. 67:40-8.
- Migliorati M, Amorfini L, Signori A, Biavati AS, Benedicenti S. (2015) Clinical and Aesthetic Outcome with Post-Extractive Implants with or without Soft Tissue Augmentation: A 2-Year Randomized Clinical Trial. Clin Implant Dent Relat Res. 17(5):983-95.
- Kan JY, Rungcharassaeng K, Lozada JL, Zimmerman G. (2011) Facial gingival tissue stability following immediate placement and provisionalization of maxillary anterior single implants: a 2- to 8-year follow-up. Int J Oral Maxillofac Implants. 26(1):179-87.
- Fickl S, Kebschull M, Schupbach P, Zuhr O, Schlagenhauf U, et al. (2011) Bone loss after full-thickness and partial-thickness flap elevation. J Clin Periodontol. 38(2):157-62.
- Bassetti RG, Stähli A, Bassetti MA, Sculean A. (2016) Soft tissue augmentation procedures at second-stage surgery: a systematic review. Clin Oral Investig. 20(7):1369-87.
- Misch CE, Al-Shammari KF, Wang HL. (2004) Creation of interimplant papillae through a split-finger technique. Implant Dent. 13(1):20-7.
- Palacci P, Nowzari H. (2008) Soft tissue enhancement around dental implants. Periodontol 2000. 47:113-32.
- Bassetti RG, Stähli A, Bassetti MA, Sculean A. (2017) Soft tissue augmentation around osseointegrated and uncovered dental implants: a systematic review. Clin Oral Investig. 21(1):53-70.
- Rojo E, Stroppa G, Sanz‐Martin I, Gonzalez‐Martín O, Nart J. (2020) Soft tissue stability around dental implants after soft tissue grafting from the lateral palate or the tuberosity area – A randomized controlled clinical study. J Clin Periodontol. 47(7):892-99.
- Rojo E, Stroppa G, Sanz-Martin I, Gonzalez-Martín O, Alemany AS, et al. (2018) Soft tissue volume gain around dental implants using autogenous subepithelial connective tissue grafts harvested from the lateral palate or tuberosity area. A randomized controlled clinical study. J Clin Periodontol. 45(4):495-03.
- Roccuzzo M, Gaudioso L, Bunino M, Dalmasso P. (2014) Surgical treatment of buccal soft tissue recessions around single implants: 1-year results from a prospective pilot study. Clin Oral Implants Res. 25(6):641-46.

