Mccune Albright Syndorme/Craniofacial Fibrous Dysplasia and Postcraniectomy Syndrome: Case Report and Literature Review
Jefferson Arce Tovar1, Erick E Muñoz Rodríguez2*, Jhonier Osorio Valencia3, Daniela Toro3 and Luis F Duarte Correales4
1Physician, School of Medicine and Health Sciences, Universidad Militar Nueva Granada, Bogotá, Colombia 2Neurosurgery Department, Hospital Militar Central, Bogotá, Colombia
3Sixth year medical student, School of Medicine and Health Sciences, Universidad Militar Nueva Granada, Bogotá, Colombia
4Sixth year medical student, Universidad El Bosque, Bogotá, Colombia
*Corresponding author: Erick E Muñoz Rodríguez, Neurosurgery Department, Hospital Militar Central, Bogotá, Colombia
Citation: Tovar AJ, Rodriguez MEE, Valencia JO, Toro D, Duarte Correales LF. (2022) Mccune Albright Syndorme/Craniofacial Fibrous Dysplasia and Postcraniectomy Syndrome: Case Report and Literature Review. Adv Clin Med Res. 4(1):1-13.
Received: November 8, 2022 | Published: January 15, 2023
Copyright© 2023 genesis pub by Tovar JA, et al. CC BY-NC-ND 4.0 DEED. This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International License.,This allows others distribute, remix, tweak, and build upon the work, even commercially, as long as they credit the authors for the original creation.
DOI: https://doi.org/10.52793/ACMR.2023.4(1)-46
Abstract
Introduction: McCune Albright syndrome (MAS) is a rare pathology caused by a genetic mutation of the GNAS1 gene that causes fibrous dysplasia (FD) among other endocrinological and metabolic manifestations. Here is presented a case report, in which craniofacial dysplasia (CFFD) produces functional alteration, its treatment, and the management of underlying complications.
Case Report: A 10-year-old female patient who presented with CFD with progressive involvement of the orbital cavity, compression of the optic nerve and right oculomotor cranial nerves, associated with precocious puberty and the appearance of ‘’café-au-lait’’ spots on the skin. ', finally, the diagnosis of MAS is made. Right front-parieto-temporal craniectomy and intra canalicular decompression of the optic nerve was performed getting partial improvement of functional alteration. Subsequently, cranioplasty is performed to manage post craniectomy/trephined syndrome.
Discussion: MAS is a genetic postzygotic disease that occurs in early stages of embryonic development, which explains its mosaicism. Among its manifestation is FD, which is explained by the hyperfunctioning nature of the mutation. In the case of DCF, continuous surveillance is required by a multidisciplinary team that includes a neurosurgeon, whose intervention is reserved for cases in which functional alterations occur, following the recommendations given by medical literature regarding the approach and type of surgery. Decompressive craniectomy can be associated with complications such as post craniectomy/trephined syndrome, which shows improvement or resolution with cranioplasty.
Conclusion: The diagnosis of MAS is clinical, and its mosaicism is explained by its early presentation in embryonic development. In the case of FCD with functional compromise, neurosurgical intervention is required, seeking the recovery and preservation of the compromised cranial nerves, avoiding prophylactic decompressions. In case of complications such as postcraniectomy/trephination syndrome, timely cranioplasty should be performed as it turns out to be highly effective as a treatment, however, the need for studies aimed at its characterization and diagnosis arises.
Keywords
McCune albright syndrome; Fibrous dysplasia; Craniofacial fibrous dysplasia; Craniofacial surgery; Post craniectomy syndrome; Trephined syndrome; Cranioplasty
Introduction
McCune Albright syndrome is a rare pathology caused by a post zygotic genetic mutation of the GNAS1 gene that classically causes FD, hyper functioning endocrinepathies and ‘’café-au-lait’’ spots on the skin, that make up the classic triad of its presentation, although, other hormonal and metabolic alterations are currently described. Despite its infrequency, it is a condition that represents a challenge for the multidisciplinary team that must evaluate it, which includes the neurosurgeon or craniofacial surgeon when CFFD is present, so is necessary to know the recommendations and advances regarding available interventions. Likewise, the description and understanding of complications that, although rare, may occur after a craniectomy, such as post craniectomy/trephined syndrome, is required. We conducted a review of the literature regarding the report of a case of MAS with polyostotic FD, with functional complications that required de-compressive surgical management and cranioplasty was subsequently performed to manage post-craniectomy syndrome.
Case Report
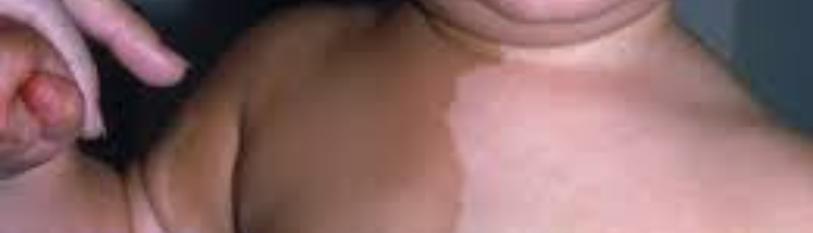
A 10-year-old female patient with a history of right frontal mild cranioencephalic trauma (MCET) who consulted 3 years later for a new MCET in the same anatomical region with evidence of front-orbital growth, in whom right upper frontal hyperostotic reaction is considered. The mother reports the larche, pubarche and apocrine odor since she was 8 years old. Physical examination reveals right frontal deformity associated with ipsilateral palpebral ptosis. Additionally, in a radiological study by simple skull computed tomography (SSCT), right front sphenoidal FD is found. Late outpatient follow-up is performed due to the Sars-Cov-2 pandemic, on clinical examination, facial asymmetry with right frontal protrusion and exophthalmos is found; visual alteration with mydriatic pupil, poor response to the direct and consensual photo motor reflexes, paresis of the IV and VI cranial nerves and partial optic atrophy with temporary pallor of the nerve, accompanied by visual acuity deterioration that progresses to amauros is, confirmed by visual campimetry. In addition, there is evidence of the appearance of ‘’café au lait’’ spots on the abdomen (Figure 1). A new SSCT with 3D reconstruction and magnetic resonance imaging (MRI) shows that the right FD progresses until it partially compromises the optic nerve and the facial and orbital cavity symmetry (Figure 2-3).
Figure 1: Abdomen’sphotograph thatshows a single café-au-lait macule on skin.
Figure 2: SSCT with 3D reconstruction. A) Superior view of the 3D reconstruction showing bone protrusion in the right frontal region that compromises facial symmetry. B) Front view showing compromised symmetry of the right orbital cavity. C) Axial SSCT slice showing obstruction and occupation of the right orbital cavity by dysplastic tissue. D) Sagittal slice with right frontoethmoidal bone expansion, with changes in bone density suggestive of fibrous dysplasia.
Figure 3: Brain MRI. A) Axial slice T1 sequence showing dysplastic tissue compromising orbital cavity. B) Sagittal T2 slice with right frontoethmoid sphenoid fibrous dysplasia and partial involvement of the right optic nerve.
Admission to an intermediate care unit is considered due to the risk of progressive intracranial hypertension. In laboratories there is evidence of vitamin D deficiency and hydroelectrolytic disorder. Differential diagnoses include neurofibromatosis, osteofibrous dysplasia, idiopathic central puberty, and the possibility of a syndromic origin. Considering polyostotic FD of craniofacial location, precocious puberty, café-au-lait spots and derived metabolic disorders, the diagnosis of McCune Albright syndrome is made.
Vitamin D supplement 2000 IU/day, calcium carbonate 1200 mg/day and start of pubertal brake with gonadotropin-releasing hormone analog (GNRH), triptorelin pamoate 11.25 mg in joint assessment by neurosurgery and pediatric endocrinology are indicated. Due to CFFD, right frontoparietotemporal de-compressive craniectomy with intra canalicular decompression of the optic nerve is considered. Surgical findings include right frontal-parietal bone dysplasia, intracranial hypertension, as well as compressed right orbital fissure and optic canal. After the intervention, improvement of cranial nerve alterations is observed without alteration of neurological status, for that reason it is considered discharge.
One year after the decompressive craniectomy, she consulted for high-intensityoppressive headache in the right parietal region, associated with ipsilateral eye pain, dizziness, and nausea. Physical examination reveals a right cranial bone defect and palpebral ptosis on the same side. Due to a history of extensive craniectomy and the appearance of neurological symptoms, she is considered to have post-craniectomy syndrome and is hospitalized. Therapy with bisphosphonates (zoledronic acid) and ionic and vitamin D supplementation is started due levels are found to be below the normal limit. Heterologous graft cranioplasty is performed without complications, with improvement of the symptoms for which she was admitted (Figure 4).
Figure 4: Axial SSCT showing bone defect prior to cranioplasty, without radiological evidence of intracranial pressure alteration. B) 3D reconstruction by computed tomography showing bone defect with small foci of dysplasia.
Discussion
Etiology and Pathophysiology
In MAS there is an increased activity of Gs protein signaling, caused by an acquired somatic gain-of-function mutation in the GNAS gene encoding its alpha subunit. G protein coupled receptors (GPCRs) participate in multiple cellular pathways, including some that have been identified as regulators of osteoblast formation and function, influencing bone formation and homeostasis[2-3]. The most accepted models and theories affirm that the mutation is postzygotic, explaining the mosaicism that characteizes the disease, also justified by the ubiquity of the protein in the affected tissues, making the range of extension and severity of its manifestation variable [2-3]. In addition, the time in which the genetic alteration occurs also explains its non-hereditary nature, due it does not compromise the germ line[2]. The involvement of tissues of the three germ layers suggests the appearance of the mutation in early stages of embryonic development, supported by models in which the alteration presents in a pluripotent stem cell ends up affecting a wide variety of tissues, resulting in the phenotype of the syndrome depends on the cell type, number of affected cells, their viability, and epigenetic factors. This variable expression also influences on the severity of the disease, leading to earlier mutations, greater extension of the disease[1-4].
Molecular-level changes include inhibition of GTPase activity and persistent stimulation of adenylate cyclase, causing persistently elevated intracellular levels of cyclic AMP and dysregulation of signaling, a consequence of specific amino acid substitution in the GTP hydrolase domain ofGs alpha subunit[2-5]. As a result of the mutation, normal bone is replaced by structurally defective osteofibrous tissue, in addition to the compromise of the hematopoietic bone marrow in relation to the affected site. Histologically, fibrous tissue is characterized by the presence of fibroblasts that express markers of early stages of osteogenic maturation, suggesting dysregulation in the differentiation of osteogenic progenitors to mature bone cells, resulting in the production of excessive amounts of abnormal bone matrix [6]. It has been found that tissue samples with FD produce elevated basal levels of IL-6, a cytokine that mediates osteoclastogenesis, causing increased bone resorption in the affected bone [4-6]. The endocrine pathways in which the signaling of their hormones and receptor proteins are ligand-dependent, such as LH, FSH, TSH, GHRH and ACTH, show hyperfunction, resulting in the characteristic endocrinological alterations of the syndrome [7-8].
Diagnosis
Clinical suspicion of this syndrome is based on the presence of FD and one or more extraskeletal characteristics of the disease; or the presentation of two or more manifestations of the entity other than FD [1] (Figure 5,6). FD is defined by the finding of expansive bone lesions that lead to fragility, malformations, and pain. Once suspected, an exhaustive evaluation is carried out that will allow its definitive diagnosis and classification [3]. Considering the extension of the skeletal lesion, there is a monostotic form in which the alteration is present in a single anatomical site or point, and another in which it is present in more than one point without extra skeletal manifestations called polyostotic [1-3].
Figure 5: Intraoperative photograph of bone defect correction with heterologous graft.
Figure 6: Classic presentation of McCune Albright syndrome.
The extraskeletal manifestations described for the diagnosis of MAS include:
- Café-au-lait macules on ''Café-au-lait'' skin: jagged and irregular edges, respect the midline.
- Gonadotropin-independent sex steroid production leading to precocious puberty causing, in addition:
- In girls, recurrent follicular cysts, alterations in menstrual bleeding.
- In boys, testicular lesions with or without precocious puberty due to autonomous testosterone production.
- Thyroid lesions with or without hyperthyroidism (non-autoimmune)
- Excess growth hormone.
- Neonatal hypercortisolism(1).
The presence of intramuscular myxomas is also considered a manifestation, which denotes Mazabraud syndrome [1]. There are cases where hypophosphatemia mediated by Fibroblast Growth Factor 23 (FGF-23) occurs, which is not considered a manifestation of the syndrome, but rather a marker of the severity of FD. The diagnosis of the syndrome is clinical, confirmed through laboratories aimed at evidencing hormonal and metabolic alterations. Molecular or histological confirmation is not required except in cases where there is doubt regarding the diagnosis or the initial analyzes are inconclusive [1-3]. There is a wide variety in the forms of radiological presentation, given the mosaicism that precedes the bone manifestations of the syndrome, in addition to the fact that its location varies between the axial skeleton, extremities and the skull. For the latter, the frequency of presentation of lesions shows the frontal bone as the most affected, followed by the sphenoid, ethmoid, parietal, temporal and occipital bones [5]. Bone expansion with the appearance of ground glass and deformities of the cranial vault leading to exophthalmos as the specific radiological characteristics of its involvement[9]. In addition, the presence of lesions with the appearance of extraosseous soft mass should be evaluated, due they suggest malignancy [1]. (Table 1) summarizes the usefulness of the studies radiological data available for the assessment of FD.
|
Radiological study |
Utility |
|
CT* |
Evaluate nerve entrapment and optic nerve compression. |
|
Three-dimensional bone reconstruction with helical CT
|
Optimal visualization of the extent of dysplastic bone in the skull base · Frosted glass pattern. · Homogeneously dense pattern. · Cystic variety. |
|
MRI † |
Image will depend on factors such as the number of bone trabeculae and degree of cellularity: Low intensity images with well-marked borders on T1 and T2. |
|
SPECT ‡ |
Sensitive in detecting the edges of the DF |
Note. *Computed Tomography, † Magnetic Resonance Imaging, ‡ Single Photon Emission Computed Tomography. Taken from Fibrous Dysplasia of the Sphenoid and Skull Base. Vol. 44, Otolaryngologic Clinics of North America. 2011.
Table 1: Recommended radiological studies and their usefulness.
The Best practice management guidelines for fibrous dysplasia/McCune-Albright syndrome: A consensus statement from the FD/MAS international consortium proposes staging to determine the extent and impact of the disease at diagnosis, to guide treatment and minimize the risk of complications. Among the key components for staging is the evaluation of CFFD, for which, in addition to the clinical history and physical examination, the assessment of: facial asymmetry using photography is recommended; psychological impact using the craniofacial experience index; radiological evaluation including fine cut computed tomography (CT) 1 mm slice tickness; added to the reference to specialties such as neurosurgery, plastic surgery, ophthalmology, maxillofacial surgery or audiology in the case of injuries adjacent to nerve pathways or relevant structures (Figure 7).
Figure 7: Diagram of suggested assessments for the follow-up of patients with CFDF according to ''The Best practice management guidelines for fibrous dysplasia/McCune-Albright syndrome: A consensus statement from the FD/MAS international consortium'' .
Treatment
The management of the manifestations of the syndrome will depend on the phenotype, having to adjust the interventions to each case. The hormonal, metabolic and structural manifestations that can occur in the disease are summarized together with their specific management, monitoring tool and objective (Table 2).
|
Manifestations |
Elements to evaluate |
Intervention or management |
Objective |
|
FGF-23-induced renal phosphate wasting |
|
Active metabolite or analogue of vitamin D: calcitriol 1 μg/day. Phosphate supplementation 15-60 mg/kg/day in 4-5 doses |
Adjust vitamin D and phosphate to suppress hyperparathyroidism and maintain urinary calcium below the upper limit |
|
Scoliosis |
|
Early follow-up with spine team and physiotherapist. Consider surgical fixation if Cobb angle >30º |
Stop progression and secondary functional alteration |
|
Bone pain |
|
If it is secondary to fractures, carry out the corresponding surgical management. Analgesic management as needed.
|
Remission of symptoms |
|
Ovarian/testicular pathology |
Ovarian:
|
Avoid surgical management, indicated only when there is a risk of torsion. Precocious puberty treatment if bone age is advanced and there is frequent bleeding. Avoid contraception with estrogenic components
|
Stop precocious puberty and alterations of the menstrual cycle. Decrease the risk of breast cancer from prolonged exposure to estrogens
|
|
Testis:
|
Avoid surgical management. Treatment of precocious puberty. Annual follow-up for testicular lesions and biopsy in case of change in lesions’ size. |
Management of precocious puberty. Surveillance and control of testicular lesions in case of suspected malignancy |
|
|
Thyroid pathology |
Hyperthyroidism:
|
Short-term: pharmacological antithyroid management > 5 years: Thyroidectomy, radioablation with annual follow-up. Patients under 10 years of age with ultrasound and abnormal thyroid function: Follow-up every 6-12 months. |
Normalization of thyroid function and injury surveillance |
|
Excess growth hormone |
|
First line: Somatostatin analogs. Patients resistant to medical therapy: Hypophysectomy (complicated in patients with CFFD) Pituitary radiation
|
IGF-1 Z score between -2 and +1. Growth velocity: Annual Head circumference, IGF-1: Growing children |
|
Adrenal pathology |
|
Management: Metyrapone + Etomidate or Ketoconazole. Bilateral adrenalectomy in critically ill patients |
Surveillance in case of spontaneous resolution or prolonged hypercortisolism |
Note: Taken from ''The Best practice management guidelines for fibrous dysplasia/McCune-Albright syndrome: A consensus statement from the FD/MAS international consortium''.
Table 2: Overview of MAS clinical manifestations.
(See table 2. Document) For the management of CFFD, a control of the elements present in the diagnosis of the syndrome must be achieved before carrying out an intervention [3]. Active surveillance is suggested and a balance between the risks and benefits of resection and/or reconstruction is suggested, considering, among other things, the tendency of fibrous tissue to stabilize after adolescence, justifying those interventions be delayed until puberty [10]. The goals of treatment are listed below:
- Prevention of functional loss, especially vision and hearing.
- Stop or reduce physical malformation.
- Prevention of secondary deformities.
- Minimize long-term morbidity.
- It is recommended to avoid confirmatory biopsies or surgery in asymptomatic patients, in addition to performing periodic clinical and radiological controls. Symptoms that should be part of the active search in patients with CFFD include cranial neuropathies, pain, and malformations [3,7]. For proper follow-up, check-ups with the craniofacial surgery team should be at least once a year. Radiological surveillance is performed with CT, which is suggested every two years in children and every 5 years in asymptomatic adults, although, the frequency of clinical and imaging controls also depends on the extension and the risk of complications. Hearing evaluation should be performed annually in patients with skull base malformations. Given the evidence of functional compromise, assessment and intervention by skull base surgery should be considered [3]. Depending on the location of the lesion and the objectives to be achieved, the surgical treatment options vary, among which are: burring, which objective is to achieve symmetry and minimize the volume of the deformity; and subtotal or total excision with subsequent reconstruction of the defect. In lesions of the frontal region that can affect the orbital morphology deforming the positioning of the eyeball, preoperative evaluation by ophthalmology is suggested, considering the possibility of diplopia associated with the surgical intervention. For the naso-ethmoid region, reducing airway obstruction adds to the purpose of management. Prophylactic decompression of the optic nerve is not recommended in any case [3-5] [11-12].
For the choice of the type of intervention, extensive excision of the dysplastic tissue is recommended since it has been associated with a lower rate of recurrence. A more conservative management, seeking the reduction and contouring of the affected facial bones, has shown a greater recurrence, so it is not recommended for these patients. The surgical approach is mainly determined by the location of the disease and can be anterior, pterional or endonasal, mainly for isolated lesions of the ethmoid, sphenoid or frontal [5].
Postcranectomy Syndrome
Post-craniectomy syndrome, trephination syndrome or sunken skin flap syndrome is defined as neurological deterioration or dysfunction after craniectomy, which symptoms worsen when the patient is positioned vertically and improve or disappear once cranioplasty is performed[13]. Its pathophysiology has not been established; however, some studies maintain that elements such as the reduced volume of cerebrospinal fluid, the action of atmospheric pressure that, in the absence of a segment of the cranial vault, alters the content/container balance and leads to changes in intracranial pressure, the amplitude of the craniectomy and the period between it and the cranioplasty participate in the development of the clinical manifestation[13-15]. Its diagnosis supposes the precedent of craniectomy accompanied by neurological symptoms such as motor weakness, cognitive deficit, language deficit, altered level of consciousness, headache, seizures, cranial nerve alterations, psycho-emotional symptoms, in a patient who has not undergone cranioplasty. The exacerbation of these symptoms seems to be related to postural changes, especially those that involve standing vertically, as well as the progressive sinking of the skin flap [15-18].
Despite the relationship that can be established between the appearance of symptoms and the history of craniectomy, it is believed that the syndrome is under diagnosed due to the dissociation between the manifestations and the radiological findings [13]. Findings such as ''paroxysmal'' herniation, deviation of structures from the midline and sunken flap have been reported as non-specific for this syndrome. On the other hand, considering the established relationship between the pressure and volume of cerebrospinal fluid and its pathophysiology, there could be a greater risk of developing alterations in patients with pressure changes in the third ventricle [14]. Its treatment is cranioplasty, before which the symptoms improve, as well as elements such as cerebrospinal fluid pressure at the lumbar level and cerebral perfusion pressure return to normal levels, suggesting a causal relationship to the defect and its change[15-19]. In the presence of hydrocephalus after craniectomy, the shunt does not seem to represent an improvement in patients, instead leading to greater neurological deterioration that is based on the understanding of the patho-physiology of the syndrome that we have so far [16].
Conclusion
MAS is a rare disorder caused by a genetic mutation that generates skeletal, metabolic, endocrinological alterations and dermatological findings that classically characterize the disease. Its manifestations are explained jointly by the ubiquity of the receptor protein that is affected and the mutation in early embryonic stages, which lead to its characteristic mosaicism. The manifestations of this disease allow a fundamentally clinical diagnosis, with the support of paraclinical studies aimed at the treatment and follow-up of its complications, such as fibrotic dysplasia. CFFD requires multidisciplinary management in which regular monitoring is the best indication for treatment, mainly of the nerve pathways that involve hearing and vision. In cases where there is functional compromise, surgical intervention options should be quickly evaluated, depending on the extent and severity of the injury, considering that subtotal/total resection and reconstruction have been shown to have better results in this group of patients. Prophylactic decompression of the optic nerve has not been shown to represent a greater benefit as reported in the literature.
Despite being a more widely described complication after trauma, tumors or vascular injuries that require craniectomy, a case of post-craniectomy/trephination syndrome after decompression with a different surgical indication is reported. The diagnosis of this condition should be suspected in any patient who has undergone a craniectomy and then presents the symptoms that define it. The absence of radiological signs should not be a criterion to rule out or delay management. Cranioplasty shows in our patient and in the systematic reviews consulted a significant partial or total improvement of the manifestations. Considering the lack of methodologically complete studies regarding the definition of radiological criteria and different elements evaluated in the syndrome, it is necessary to work out on them.
References
- Boyce AM, Collins MT. (2020) Fibrous dysplasia/McCune-albright syndrome: A rare, mosaic disease of Gαsactivation. Endocr. Rev. 41(2):345–70.
- Lung H, Hsiao EC, Wentworth KL. (2020) Advances in Models of Fibrous Dysplasia/McCune-Albright Syndrome. Front Endocrinol (Lausanne). 24:10:925.
- Javaid MK, Boyce A, Appelman-Dijkstra N, Ong J, Defabianis P, et al. (2019) Best practice management guidelines for fibrous dysplasia/McCune-Albright syndrome: A consensus statement from the FD/MAS international consortium. Orphanet J Rare Dis.14(1):139.
- Hartley I, Zhadina M, Collins MT, Boyce AM. (2019) Fibrous Dysplasia of Bone and McCune–Albright Syndrome: A Bench to Bedside Review. Calcif Tissue Int. 104(5): 517-29.
- Amit M, Fliss DM, Gil Z. (2011) Fibrous Dysplasia of the Sphenoid and Skull Base. Otolaryngol Clin North Am. 44(4):891–2.
- Riminucci M, Kuznetsov SA, Cherman N, Corsi A, Bianco P, et al. (2003) Osteoclastogenesis in fibrous dysplasia of bone: In situ and in vitro analysis of IL-6 expression. Bone. 33(3):434–42.
- Spencer T, Pan KS, Collins MT, Boyce AM. (2019) The Clinical Spectrum of McCune-Albright Syndrome and Its Management. Horm Res Paediatr. 92(6):347-56.
- Brzica K, Simunovic M, Ivancic M, Tudor D, Skrabic I, et al. (2022) McCune-Albright Syndrome in Infant with Growth Hormone Excess. Genes (Basel). 13(8):1345.
- Fitzpatrick K, Taljanovic M, Speer D, Graham A, Jacobson J, et al. (2003) Imaging Findings of Fibrous Dysplasia with Histopathologic and Intraoperative Correlation. AJR Am j Roentgenol. 182(6):1389–98.
- Mierzwiński J, Kosowska J, Tyra J, Haber K, Drela M, et al. (2018) Different clinical presentation and management of temporal bone fibrous dysplasia in children. World J Surg Oncol. 16:5.
- Cutler CM, Lee JS, Butman JA, Fitzgibbon EJ, Kelly MH, et al. (2006) Long-term outcome of optic nerve encasement and optic nerve decompression in patients with fibrous dysplasia: risk factors for blindness and safety of observation. Neurosurgery. 59(5):1017-18.
- Amit M, Collins MT, Fitz Gibbon EJ, Butman JA, Fliss DM,et al. (2011) Surgery versus watchful waiting in patients with craniofacial fibrous dysplasia - a meta-analysis. PLoS One. 6(9):e25179.
- Yoshida K, Toda M, Yamada Y, Yamada M, Yokoyama Y, et al. (2020) Sinking skin flap syndrome visualized by upright computed tomography. Acta Neurochir [Wien]. 162(8) :1825–28.
- Vasung L, Hamard M, Soto MCA, Sommaruga S, Sveikata L, et al. (2016) Radiological signs of the syndrome of the trephined. Neuroradiology. 58(6):557–68.
- Ramos-Zúñiga R, Mares-Pais R, Gutiérrez-Avila O, Saldaña-Koppel D. (2016) Paradoxical Herniation in the Postcraniectomy Syndrome: Report and Literature Update. J Neurol Surg Rep.77(1):e035–e038.
- Ashayeri K, Jackson EM, Huang J, Brem H, Gordon CR. (2016) Syndrome of the Trephined: A Systematic Review. Neurosurgery. 79(4):525–34.
- Annan M, de Toffol B, Hommet C, Mondon K. (2015) Sinking skin flap syndrome (or Syndrome of the trephined): A review. Br J Neurosurg, 29(3):314-18.
- Mustroph CM, Stewart CM, Mann LM, Saberian S, Deibert CP, et al. (2022) Systematic Review of Syndrome of the Trephined and Reconstructive Implications. J Craniofac Surg. 33(6):e647–52.
- Jeyaraj P. (2015) Importance of Early Cranioplasty in Reversing the “Syndrome of the Trephine/Motor Trephine Syndrome/Sinking Skin Flap Syndrome.” J Maxillofac Oral Surg. 14(3):666-73.

