Multicystic Ameloblastoma: Presentation of a Case with Resolution through the Fabrication of a 3D Titanium Prosthesis
Guillermo Sica1, Emiliano Lopez Moris2, Sebastian Miguelez3, Christian Oscar Mosca4 and Eduardo Rey5*
1Dentist, Specialist in Oral and Maxillofacial Surgery, Professor of the Specialization in Maxillofacial Surgery at FOUNNE, Member of the Head and Neck Service at the Trinidad and Miter Sanatorium, Buenos Aires, Argentina
2Head and Neck Surgeon, Member of the Argentinian Association of Surgery, Argentina
3Dentist at the Hospital Interzonal General de Agudos Pte. Peron Specialist in Oral Maxillary Prosthesis, Argentina
4Dentist, Specialist in Maxillofacial Surgery, Doctor in Public Health, Assistant Professor of the Microbiology and Immunology Subject, Kennedy University, Teaching Advisor of the Hospital, Pte Peron, Avellaneda, Buenos Aires, Argentina
5President of the National Academy of Dentistry; Consultant to the National Academy of Medicine; Former Professor of Oral and Maxillofacial Surgery I and II School of Dentistry University of Buenos Aires, Argentina
*Corresponding author: Eduardo Rey, President of the National Academy of Dentistry; Consultant to the National Academy of Medicine; Former Professor of Oral and Maxillofacial Surgery I and II School of Dentistry University of Buenos Aires, Argentina
Citation: Sica G, Moris EL, Miguelez S, Mosca CO, Rey E. (2021) Multicystic Ameloblastoma: Presentation of a Case with Resolution through the Fabrication of a 3D Titanium Prosthesis. J Oral Med and Dent Res. 2(1):1-11.
Received: January 15, 2021 | Published: February 15, 2021
Copyright© 2021 genesis pub by AlHayyan WA, et al. CC BY-NC-ND 4.0 DEED. This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-No Derivatives 4.0 International License., This allows others distribute, remix, tweak, and build upon the work, even commercially, as long as they credit the authors for the original creation.
2021 Genesis Pub by Sica G, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC BY 4.0). This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author(s) and source are properly credited.
Abstract
Some Ameloblastomas are benign but turn out to be recurrent tumors, the importance of which lies in their potential to grow to enormous size with consequent bone deformity. At the level of reconstructive options for mandibular continuity defects produced by these entities, the use of free microvascular flaps, free bone graft alloplastic implants, including titanium reconstruction plates and titanium trays, have been considered. In this article we will develop the situation of a 30-year-old patient who suffered from a multicysti came loblastoma that was resected with a safety margin and personalized 3D titanium prosthesis was made.
Keywords
Multicysti; Loblastoma; 3D prosthesis; Tumor of the jaws; Maxillary tumor resection.
Introduction
In 1868 Broca described the first report of ameloblastoma in the scientific literature, then Falkson completed the first detailed histological description in 1879 and Malassez in 1885 introduced the term adamantinoma, which was later abandoned. Since then, numerous synonyms have been used to refer to these tumors, up to the current name of ameloblastoma [1]. Ameloblastomas are benign, recurrent tumors, the importance of which lies in their potential to grow to enormous size with consequent bone deformity. They are generally classified into unicystic, multicystic, peripheral and malignant subtypes [1,2]. Solid multicystic ameloblastoma is a benign epithelial tumor of odontogenic origin that shows a strong tendency to recurrence and local aggression [3,4].
It is a tumor derived from the residual epithelial components of tooth development, such as: remains of the dental lamina (Serres remains), reduced enamel epithelium, Malassez remains and the basal cells of the maxillary superficial epithelium [5]. In general, its appearance is manifested between the third to the seventh decade of life, except for the unicystic variety, which is diagnosed between the second and third decade, with no differences between the sexes (6). Its manifestation is more frequent in the mandible (85%) than in the upper jaw (15%). In the mandibular location, the premolar, molar and ascending ramus of the mandible are more frequently affected, while in the upper jaw they are concentrated in the molar area, where they tend to extend to the maxillary sinus and the floor of the nostrils [6].
Mandibular masses manifest with malocclusion, loss of teeth, periodontal disease, and facial deformations [7]. At the level of reconstructive options for mandibular continuity defects, they have included the use of microvascular free flaps, free bone grafting and alloplastic implants, including titanium reconstruction plates and titanium trays [8-11]. With the establishment of these reconstructive options, advances in three-dimensional (3D) imaging software, virtual surgical planning (VSP), and computer-aided design/computer-aided manufacturing (CAD/CAM) have further improved the planning and application of these options in the restoration of mandibular defects [12].
The advantages of this technology even include visualization of tumor margins, definition of surgical margins, manufacture of surgical templates or cutting guides, evaluation of continuity defects and identification of the ideal dimensions and shape of the bone for its reconstruction [13]. In this article we will develop the situation of a patient who presented with a multicystic ameloblastoma that was resected with a safety margin and a personalized 3D titanium prosthesis was made.
Materials and Methods
For this study, the rights of the patient were protected, under the consent signed by the same, respecting the ethical principles based on the Declaration of Helsinki.
Clinical Situation
A 30-year-old female patient, referred from her dentist for presenting an increase in volume in the region of the left hemi maxilla, presented to the consultation at the Trinidad de Quilmes Clinic. A medical history and anamnesis of the patient were taken in which she manifested painless and feverish swelling. In the intra and extra oral examination, an increase in volume was observed in the bottom of the sulcus and left hemi maxillary of solid consistency, blurred edges of approximately 5 cm diameter. A routine orthopantomography was performed (Figure 1) in which a radiolucent lesion with radiopaque areas can be observed, giving the image of multicystic or multilocular.
Figure 1: Pre-surgical panoramic radiograph. In it, a lesion with multicystic characteristics can be seen that ranges from distal 36 to the entire ascending branch.
A computed axial tomography was performed, observing a radiolucent image with other radiopaque images of approximately 6 cm in diameter in the body and left maxillary branch (Figure 2a). The 3D reconstruction of the computed axial tomography shows the characteristics of the multi locular lesion of approximately 6 cm in diameter that invades from 36 to the ascending branch (Figure 2b).
Figure 2a: Computed axial tomography was performed, observing a radiolucent image with other radiopaque images of approximately 6 cm in diameter in the body and left maxillary branch.
Figure 2b: Computed Axial Tomography and 3D reconstruction. The size of the lesion can be visualized, covering tooth 36 from distally and occupying almost the entire volume of the ascending branch.
Pre-surgical studies for biopsy are requested, with the result of Ameloblastoma. In our clinical situation, a custom 3D titanium prosthesis was designed from an alloy called the alloy is Ti-6Al-4V. The head of the component is golden since a coating with titanium nitride is applied, in order to improve the hardness of the piece in its articular part and reduce wear with the passage of cycles.
The patient's computed tomography was used to generate a 3D recreation model (Figure 3a). The design of the personalized prosthesis (Figure 3b) was made virtually with the 3D printed cutting guides, fossa templates to obtain condylar resection (Figure 3c) and a better fit of the prosthesis and template of prosthesis to position the holes of the titanium prosthesis. During surgery, the occlusion was ensured.
Figure 3a: The patient's computed tomography was used to generate a 3D recreation model.
Figure 3b: The design of the personalized prosthesis.
Figure 3c: Prosthesis design, glenoid and condylar cavity template.
Surgical Intervention
The patient was admitted to the operating room, and general anesthesia was performed with naso-tracheal intubation, she was positioned in hyperextension by placing a roll at the level of the shoulder blades and rotating the head to the right. Subsequently, antisepsis of the operative field, upper third and middle region of the face, suprahyoid region and neck was performed, with 10% iodopovidone and placement of surgical fields. Comb wires were placed and intermaxillary locking was carried out to preserve the dental occlusion of the right hemimaxilla and correctly place the titanium prosthesis on the side to be resected.
Taking precautions from the Jaffe Nerve, it was approached under a semi lunar incision of about 8 cm in length at 3 cm below the rim of the mandible with a cold scalpel type bad-parker n °3 blade n °15 and electrosurgical knife to generate hemostasis. Skin, subcutaneous cellular tissue is incised, up to the plane of the cutaneous muscle or platysma. In this step, hemostasis of said subcutaneous tissue is carried out, said muscle is sectioned and a skin flap is obtained, visualizing the Jaffe nerve.
Subsequently, it is introduced to the submaxillary gland compartment, detecting the sternocleidomastoid muscle from behind, the parotid gland below the intermediate tendon and anterior belly of the digastric muscle and vessels of the vein and facial artery region that are ligated with flax 100.
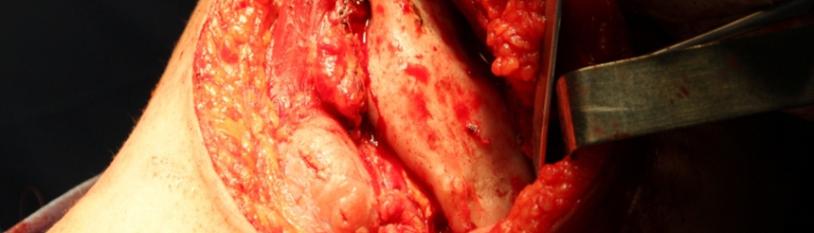
Once the triangular muscle of the lips and facial vessels are exposed, the periosteum of the mandibular body and section of the masseter muscle are sectioned, in this way the branch is exposed to the sigmoid notch, exposing the premolar teeth in front of the mental nerve and chin (Figure 4). A section mark of the mandibular bone was made at 1 cm and a half in front of the anterior edge of the tumor using the previously made 3D guide (Figure 5a, 5b), involving teeth 36 and 35. The entire disclosure was carried out. The lingual aspect of the maxilla to cure it from the floor of the mouth and the coronoid process.
Figure 4: Visualization of the surgical planes, exposing the left hemimaxilla. It is possible to observe the blowing of the body and ascending branch by the pathological entity.
Figure 5a: A section mark of the mandibular bone was made at 1 cm and a half in front of the anterior edge of the tumor using the previously made 3D guide, involving teeth 36 and 35.
Figure 5b: Use of the 3D ostectomy guide.
Subsequently, a linear left preauricular incision was made, incising skin and subcutaneous cellular tissue with hemostasis of cellular vessels until reaching the parotid plane. A flap was made in front of the temporomandibular joint forward to the tubercle of the sigmoid root, the anterior limit of said joint, to debride with hemostatic forceps in front of the superficial temporal vessels to the capsule.
These compartments are communicated with disclosure with gauze, releasing the condyle of the maxilla and proceeding to the exeresis of the lower hemimaxilla with hemostasis control. Then the silicone glenoid cavity was placed (Figure 6a,6b) and screw fixation to the zygomatic arch, and a 3D titanium preformed prosthesis was placed in replacement of the resected bone (Figure 7a,7b). Finally, it was sutured by planes with 3-0 vicryl and the skin with 4-0 nylon.
Figure 6a: The silicone glenoid cavity was placed.
Figure 6b: Location of the condylar template with Ø 2.0 to 2.3 mm screw housing.
Figure 7a: Screw fixation to the zygomatic arch, and a 3D titanium preformed prosthesis was placed in replacement of the resected bone
Figure 7b: Prosthesis made of titanium, with socket for screws Ø 2.3 to 2.8 mm.
The bone fragment corresponding to the lower jaw measures 9.5 x 3.6 x 1.5cm (Figure 8). An analgesic-anti-inflammatory drug, ketorolac 30 mg, betamethasone and hydrocortisone, and the antibiotic ampicillin with sulbactam were administered intravenously. The patient remained in the ICU (intensive care unit) for 24 hours.
Figure 8: Vestibular and lingual view of the mandibular resection fragment.
Post Surgical Control
Hospital discharge was indicated at 6 days and post-surgical measures were given in verbal and written form, outpatient medication and schedule of controls. In the controls the patient showed a very good evolution.
Pathological Result
The sections show bone fragments of the lower jaw with central infiltration due to proliferation of monomorphic epithelial cells of lobulated and plexiform arrangement, with peripheral nuclear palisade and inverted polarity associated with central cellular stellate reticulum. Acantomatous foci coexist with benign squamous cells and areas with lax stroma. Remaining cortical and trabecular bone tissue with foci of disintegration and reparative changes. Histopathological characters corresponding to bone infiltration of the lower jaw due to ameloblastoma, with a follicular and plexiform pattern. Lesion-free resection margins. Continuous postoperative controls were performed on the patient, the last one at 8 months, presenting a very good clinical evolution (Figure 9,10).
Figure 9: Clinical control of the patient 6 months after the intervention. A slight asymmetry can be observed at the facial level.
Figure 10: Panoramic control radiograph 6 months after the intervention.
Discussion
Ameloblastomas generally present asymptomatic, and small lesions are only detected during radiographic examination [1,7]. They present clinically with pain caused by edema in the upper jaw or mandible. The tumor can grow slowly to massive proportions. Paresthesias are not common, even with large tumors [14]. Root resorption does not occur very often, but is sometimes seen in some rapidly growing lesions [15]. In the case presented, the clinical findings were the presence of an asymptomatic tumor of progressive and slow growth, with facial asymmetry caused by bone expansion in the affected area, corresponding with the characteristics of this type of tumor.
The definitive diagnosis of ameloblastomas is based on the pathological analysis, as in our case presented. The differential diagnosis described in the literature mainly includes calcifying epithelial odontogenic tumor, odontogenicmyxoma, dentigerous cyst and keratocyst [16]. It is also important to differentiate between the multicystic and unicystic types, as this is the basis for the difference in prognosis. The diagnostic images allow establishing the differentiation between these two types in an adequate way. Computed tomography would allow presumptive assessment of the type of lesion, as well as observing the degree of invasion and bone involvement [17,18].
Depending on the type of ameloblastoma, Nakamura et al. [19,20] describe conservative treatment, such as marsupialization and enucleation followed by adequate bone curettage, it was very efficient, reducing the need for surgical resection. However, each case of ameloblastoma must be analyzed individually and meticulously, prioritizing, in cases where the tumor occurs in the initial stages, for a conservative treatment, even if they present a higher risk of recurrence. Such behavior, despite the controversy, is considered valid, because the complications and sequelae of these conservative surgeries are much lower than those caused by radical surgery.
Thus, several treatment modalities have been proposed for ameloblastoma, such as cryosurgery, electrocoagulation, sclerotherapy, and radiotherapy. The addition of liquid nitrogen spray through cryosurgery has reduced the recurrence rate to 30%, this is due to the ability to devitalize the bone to a depth of 1 to 2 cm, resulting in less postoperative morbidity. With regard to radiotherapy, its use has been restricted and it may be indicated in inoperable cases, since ameloblastoma is, in turn, radio resistant [21]. The treatment plan must include the rehabilitation of the patient to restore functional, anatomical and aesthetic capacity [18].
In our case presented, it is a multicystic ameloblastoma. The protocol used for the surgical treatment was complete resection of the lesion, with safety margins of ± 1 cm. Computed axial tomography of the patient was used to generate a 3D recreation model and custom titanium prosthesis was designed.
The importance of designing a medical implant for a mandibular prosthesis directly affects the personal satisfaction of the patient, the result of each implant being different depending on the patient's condition. A person, who suffered an extreme injury, presenting an unforeseen variation from normal, can benefit from a prosthesis, which is precisely planned and customized for their anatomy [22-26].
Therefore, making a prosthesis, which is biocompatible and restores facial function and aesthetics, would be an important step in the health of our patients. Precise reproduction of the mandible after resection is essential for retaining the structural anatomy of the jaw in patients and restoring facial appearance and oral cavity. The function can greatly improve the quality of life of patients. In general, reconstruction is performed regularly in patients with malignant tumors or in poor physical condition [13].
Conclusion
Due to the growth of the lesion and its biological behavior outside normal parameters, in the present case the most feasible option was to carry out a total resection of the lesion in order to avoid or reduce the possibility of recurrence, followed by reconstruction with osteosynthesis prosthesis designed in 3D to restore functionality to the temporomandibular joint and the mandibular bone, without neglecting the aesthetics of the patient.
The demand for 3D printing is increasing and will become more widespread in surgical use in the near future as physicians and engineers are increasingly using technology with flexibility in the production of different medical items [27-29].
References
- Mosca C, Miguelez S, Rey E. [2020] Tumores odontogenicos benignos. In Rey EAR. Cirugia bucomaxilofacial. CABA: Corpus . Pp. 205-13.
- Gupta N, Saxena S, Rathod V, Aggarwal P. [2011] Ameloblastomauniquistico de la mandibula. Revista de Patologia Oral y Maxilofacial. 15(2):228-31.
- Barnes L, Everson J, Reichart P, Sindransky D. [2005] Clasificacion de tumores de la OMS”, en Patologia y genetica de la cabeza y el cuello. IARC Press, Lyon, Francia. Pp. 296-300.
- Speight PM, Takata. [2017] Classification of odontogenictumours (New tumour entities in the 4th edition of the World Health Organization Classification of Head and Neck tumours: odontogenic and maxillofacial bone tumors. 4th Edition.
- Philip J, Eversole L, Wysocki G. [1998] Patologia oral y maxilofacial. Madrid: Harcourt. Pp. 128-34.
- Lopez Alvarengaa R, Chrcanovic BR, Horta MC, Souza LN, Freire-Maia B. [2010] Ameloblastomamultiquistico mandibular tratado con terapiamenosinvasiva: Casoclínico y revisión de la literatura. REV ESP CIR ORAL MAXILOFAC. 32(4):172-77.
- Curi M, Dib L, Pinto D. [1997] Management of solid ameloblastoma of the jaws with liquid nitrogen spray cryosurgery. Oral Surg Oral Med Oral Pathol. 84:339-44.
- Disa J, Cordeiro P. [2000] Mandible reconstruction with microvascular surgery. Semin Surg Oncol. 19(3):226-34.
- Mertens C, Decker C, Engel M, Sander A, Hoffmann J, et al. [2014] Early bone resorption of free microvascularreanastomized bone grafts for mandibular reconstruction-a comparison of iliac crest and fibula grafts. J Craniomaxillofac Surg. 42(5):e217-e223.
- Handschel J, Hassanyar H, Depprich R. [2011] Non-vascularized iliac bone grafts for mandibular reconstruction-requirements and limitations. In Vivo. 25(5):795-99.
- Tideman H, Samman N, Cheung L. [1998] Functional reconstruction of the mandible: a modified titanium mesh system. Int J Oral Maxillofac Surg. 27(5):339-45.
- Ow A, Tan W, Pienkowski L. [2016] Mandibular Reconstruction Using a Custom Made Titanium Prosthesis: A Case Report on the Use of Virtual Surgical Planning and Computer Aided Design/Computer-Aided Manufacturing. Craniomaxillofac Trauma Reconstr. 9(3):246-50.
- Kozakiewicz M, Wach T, Szymor P. [2017] Two different techniques of manufacturing TMJ replacements - A technical report. J Cranio-Maxillo-Fac Surg. 45(9):1432-7.
- Neville B, Damm D, Allen C, Bouquot J. [2002] Oral and maxillofacial pathology. 2 Edn. Filadelfia: Saunders. Pp. 586-94.
- Borrello R, Bettio E, Bacci C, Valente M. [2016] A conservative approach to a peripheral ameloblastoma. Case Rep Dent. 82:545-71.
- Urbano del Valle S, Tovio Martinez E, Lopez Aparicio E. [2018] Ameloblastomamultiquistico de crecimiento rapido con reconstrucción parcial. Revista Cubana de Estomatologia. 55(4):1-8.
- Ferreti C, Polakow R, Coleman H. Recurrent ameloblastoma: Report of 2 cases. J Oral Maxillofac Surg. 2000; 58: p. 800-4.
- de Aguiar S, Carneiro S, Cavalcanti de Egito Vasconcelos B, de Socorro Orestes M, Figueiredo Leal J, et al. [2011] Hemi-mandibulectomia como tratamiento de Ameloblastoma Multiquistico. Acta Odontologicavenezolana. 49(3):33-34.
- Nakamura N, Higuchi Y, Tashiro H, Ohishi M. [1995] Marsupialization of cystic ameloblastoma: a clinical and histopathologic study of the growth characteristics before and after marsupialization. J Oral Maxillofac Surg. 53(7):748-54.
- Nakamura N, Higuchi Y, Mitsuyasu T, Sandra F, Ohishi M. [2002] Comparison of long-term results between different approaches to ameloblastoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 93(1):13-20.
- Bataineh A. [2000] Effect of preservation of the inferior and posterior borders on recurrence of ameloblastoma of the mandible. Oral Surg Oral Med Oral Pathol. 90(2):155-63.
- Leandro L, Ono H, Loureiro C. [2013] A ten-year experience and follow-up of three hundred patients fitted with the Biomet/Lorenz Microfixation TMJ replacement system. Int J Oral Maxillofac Surg. 42(8):1007-13.
- Mercuri L. [2016] Temporomandibular joint total joint replacement-TMJ TJR a comprehensive reference for researchers, material scientists and surgeons. New York: Springer International Publishing.
- Kurtoglu C, Kurkcu M, Sertdemir Y. [2004] Temporomandibular disorders in patients with ray based on rapid prototyping. Medical engineering and Physics. 26:671-76.
- Chen T, Wang H, Cheng T. [2000] The rationale of mandible reconstruction in advanced oral cancer: alloplastic material versus autogenous vascularized bone graf. Mater Sci Eng. 13(1/2):49-58.
- Eggber D. [2018] Computer design of bio structures. 3D Bio-printing for Reconstructive Surgery techniques and applications. 33-73.
- Rengier F, Mehndiratta A, Teng-Kobligk H. [2010] 3D printing based on imaging data: review of medical applications. Int J Comput Assist Radiol Surg. 5:335-341.
- Fletcher J, Windolf M, Richards G. [2019] Importance of Locking Plate Positioning in Proximal Humeral Fractures as Predicted by Computer Simulations. J Orthoped Res. 37:957-964.
- Dimitroulis G, Austin S, Sin Lee P, Ackland D. [2018] A new three-dimensional, print-on demand temporomandibular prosthetic total joint replacement system: preliminary outcomes. J Craniomaxillofac Surg. 46(8):1192-8.

