Applications of Dental Stem Cells for Regenerative Medicine in Oral Surgical Procedures
Karley Bates and Vincent S Gallicchio*
1Department of Biological Sciences, College of Science, Clemson University, Clemson, South Carolina, 29636 USA
*Corresponding author: Vincent S Gallicchio, Department of Biological Sciences, College of Science, Clemson University, Clemson, South Carolina, 29636 USA.
Citation: Gallicchio VS, Bates K. (2021) Department of Biological Sciences, College of Science, Clemson University, Clemson, South Carolina, 29636 USA. J Oral Med and Dent Res. 2(1):1-18.
Received: May 13, 2021 | Published: May 31, 2021
Copyright© 2021 Genesis Pub by Bates K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC BY 4.0). This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author(s) and source are properly credited.
Abstract
The use of mesenchymal stem cells in regenerative medicine is at the forefront of both stem cell and dental research. Various tissue engineering therapies are being investigated and integrated into common oral surgical procedures. Mesenchymal stem cells are multipotent stem cells that can differentiate into many cell types, and dental mesenchymal stem cell populations have been identified from various sources in the oral cavity. Mesenchymal stem cells are being considered as a means of tissue regeneration for various craniofacial structures both in the alveolar ridge itself and in surrounding facial tissues. The aim of this review is to completely and concisely review the current clinical application and use of orally derived stem cells in oral surgery, focusing on application in a clinical setting based on the regenerative potential that these cells possess.
Keywords
Dental mesenchymal stem cells; Regenerative dental medicine; Oral surgery
Abbreviations
MSCs: Mesenchymal Stem Cells; DPSCs: Dental Pulp Stem Cells; SHED: Stem Cells From Human Exfoliated Deciduous Teeth; PDLSCs: Periodontal Ligament Stem Cells; DFSCs: Dental Follicle Stem Cells; TGPCs: Tooth Germ Progenitor Cells; GMSCs: Gingival Mesenchymal Stem Cells; ABMSCs: Alveolar Bone Mesenchymal Stem Cells; SCAP: Stem Cells From The Apical Papilla; DSSCs: Dental Socket Stem Cells; PDL: Periodontal Ligament; PRP: Platelet Rich Plasma; PRGF: Platelet Rich In Growth Factors; PRF: Platelet Rich Fibrin
Introduction
Oral health and overall health have shown to be correlated throughout an individual’s lifetime [1]. Consequently, continual developments in oral surgery for the regeneration of teeth and orofacial bone along with repaired function of these structures serve to guard against general decline in oral health and associated quality of life. Current research highlights improving therapeutic treatments of various dental, oral, and craniofacial diseases and procedures that often result in tissue loss. Regenerative medicine for the preservation of oral health is an emerging field of biotechnology that includes tissue engineering and the use of different biomaterials to regenerate, replace, or repair lost tissues. The goal of personalized medicine in general in dentistry is to incorporate individualized biomaterials that complement traditional restorative surgery techniques into common clinical practice. The reasoning behind advancing personalized medicine is to utilize the body’s natural ability to heal itself, regenerate new tissue, and produce new cells as a part of the wound healing process. Stem cell therapy for tissue regeneration is the main focus of many of these advancements in research and testing, specifically in the field of dentistry [2]. It is well known that stem cells migrate from surrounding tissues and play an important role in the healing process. Stem cells are influential to the field of regenerative medicine because they are autologous to native host tissue, pose low tumorigenesis risk, and are unlikely to be rejected by the host immune system [3].
The three main classifications of stem cells: mesenchymal stem cells, embryonic stem cells, and induced pluripotent stem cells. Mesenchymal stem cells (MSCs), also known as adult stem cell, are immature multipotent cells that have the capacity to mature, multiply indefinitely, and differentiate into various more specialized cells and tissues. These cells may originate from neural crest cells and stem cells during embryonic and postnatal development [4]. To be classified as MSCs, the cells must exhibit the capacity to both self-replicate and differentiate into multiple cell types [5]. MSCs are the most frequently researched and most clinically utilized stem cell group. As multipotent progenitors, MSCs exercise the capacity to mature and differentiate into three distinct mesenchymal tissue lineages: mesodermal, ectodermal, and endodermal lineages [6]. Additionally, MSCs are distinguished by their capability to self-renew via mitosis and proliferate to sustain the source undifferentiated stem cells. MSCs have also demonstrated various immunomodulatory functions including supporting angiogenesis, anti-inflammatory, and anti-apoptotic processes which highlight their great potential for use in regenerative medicine [2,3]. Moreover, MSCs are not extensively involved in ethical controversy and can be harvested from many adult bodily tissues and thus is the topic of this review [5-7].
The study of human MSCs started after their identification in bone marrow. Experiments have demonstrated that these bone marrow derived mesenchymal stem cells (BM-MSCs) are able to regenerate specific skeletal tissues including bone, cartilage, adipose, and fibrous tissues [8]. Research has illustrated the use of BM-MSCs to be effective in tissue regeneration in many areas of the body as a treatment for various diseases and conditions, however, the relatively invasive cellular harvesting methods make BM-MSCs an unlikely candidate for expansion and normalization of personalized medicine treatments. Consequently, subsequent scientific research has examined methods to harvest and isolate MSCs from alternative tissues in the body. MSCs have been found and isolated from variety tissues in the human body including blood [9], spleen [10], adipose tissue [11], endometrial tissue [12], salivary glands [2], umbilical cord [13], liver [14], synovial membrane and fluid [15], oral tissues [2], bone [8], muscles [16-19] and others. Contrary to BM-MSCs, MSCs from oral sources are some of the most easily accessible stem cells and can be harvested via minimally invasive procedures at different points throughout an individual’s lifetime [20]. In addition, once the cells are harvested, they can be cryopreserved and stored until they are needed. Stem cell banking is a proactive form of personalized medicine as the individual chooses to bank their cells for their future medical needs. MSCs from oral sources exhibit similar immunomodulatory characteristics as BM-MSCs [21]. This ease of accessibility has caused researchers to explore their clinical applications in regenerative medicine. This review focuses specifically on dental tissue derived stem cell therapy for oral procedures and thus, excludes other sources of stem cells found in the body as well as non-oral applications of dental tissue derived stem cells.
MSCs have been studied in other field of dentistry that do not necessarily fall under the category of oral surgery or are considered non-surgical procedures. Advancements have been made in the field of endodontics specifically in the treatment of pulpitis [22] and in the process of dentin regeneration [23]. Because they do not directly fall under the realm of oral surgery, discussion of these procedures has been omitted from this review but is still acknowledged as application of MSCs in dental procedures.
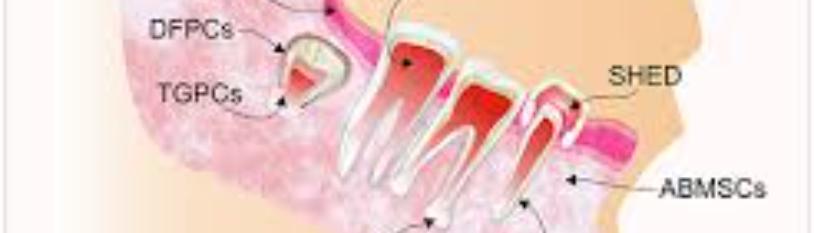
Twelve populations of MSCs have been identified and isolated in teeth and their surrounding supporting structures in the oral cavity. These sources include the following:
|
DPSCs |
Dental pulp stem cells are isolated from the dental pulps of primary or permanent teeth [24]. In vivo they have been proven to differentiate into adipocytes, endotheliocytes, and myofibers [25-27]. |
|
SHED |
Stem cells from human exfoliated deciduous teeth have been proven to differentiate into adipocytes, osteoblasts, odontoblasts, neural cells, hepatocytes, and endothelial cells [20,28]. |
|
PDLSCs |
Periodontal ligament stem cells are important in regeneration of the tissues involved with the periodontal complex [29]. In vitro they have been proven to differentiate into osteoblast-like cells, cementum tissue, Sharpey’s fibers, adipocytes, and collagen-forming cells [30,31]. |
|
DFSCs |
Dental follicle stem cells are found in the connective tissue surrounding the developing tooth germ [32]. The have been proven to differentiate into osteoblast, cementoblast, alveolar bone, dentin-like tissues, PDL, cementum, adipocyte, chondrocyte, cardiomyocyte, and neuron-like cells [31,33-35]. |
|
TGPCs |
Tooth germ progenitor cells (TGPCs) are found in the tooth germ of third molars [36]. They have been proven to differentiate into adipogenic, chondrogenic, osteogenic, odontogenic, and neurogenic like cells [2]. |
|
GMSCs |
Gingival mesenchymal stem cells of periodontium [37]. |
|
PSCs |
Periosteum stem cells are still under investigation regarding differentiation capabilities. |
|
ABMSCs |
Alveolar bone mesenchymal stem cells have been proven to differentiate into osteoblasts, adipocytes, and chondroblasts [38, 39]. |
|
SCAP |
Stem cells from apical papilla are located within immature roots of teeth and can be isolated from the immature permanent apical papilla [40-42]. SCAP have been proven to differentiate into odontogenic, chondrogenic, osteogenic, adipogenic, neurogenic, and hepatogenic cells [43]. |
|
DSSCs |
Dental socket stem cells (DSSCs) are still under investigation regarding differentiation capabilities [44]. |
|
SGSCs |
Salivary gland stem cells (SGSCs) are still under investigation regarding differentiation capabilities [35]. |
|
iPAPs |
Inflamed periapical tissues are still under investigation regarding differentiation capabilities [35]. |
MSCs as stated above exist in the oral mucosa (GMSCs, OESCs, PSCs) and have osteogenic and neurogenic differentiation capacities [35]. However, these cells are not well understood, and few stem cells from oral mucosa have been isolated to date. Additionally, while research and clinical applications exist regarding the use of many MSCs in dentistry, no information is currently available regarding OESCs [42,45]. Variation in the levels of cell proliferation and range of differentiation capability exists between these stem cell sources. Novel discoveries continue to be made about which stem cells have the most favorable qualities for success in applications in oral regenerative medicine procedures.
Some oral and maxillofacial surgical procedures that could benefit from the application of MSCs include are surgical assistance with periodontal disease, dentoalveolar surgery, surgical placement of dental implants, surgical correction of maxillofacial skeletal deformities, orthognathic surgery, cleft and craniofacial surgery, maxillofacial trauma, temporomandibular joint correction, treatment of various pathologic conditions, and reconstructive and cosmetic surgeries. Not all of these procedures have extensive research regarding the application of MSCs, but each of these surgeries require bony and soft tissue management or reconstruction therefore stem cell therapy as a component of convention treatment should be considered.
Discussion
Recent research regarding stem cell applications in oral surgical procedures focus on determining which oral source of MSCs is most effective in tissue regeneration, the ideal means of transplanting the stem cells to the treatment site, the best microenvironment for the MSCs [46], and the best growth factors to encourage proliferation and differentiation [47]. There is an enormous amount of techniques regarding cellular transplant therapies because there are no specific protocols established for which biomaterials should or should not be used. Each study or trial performed includes an old method, a combination of methods, or a new proposed method for stem cell transfer. The goal is to determine the optimal means of transplantation and combination of other biomaterials to find the ideal technique and then implement it into clinical practice after repeated human trials. Tissue regeneration is very complex and difficult to study due to the many factors that can affect the success of regeneration. Current therapies for reconstruction of craniofacial defects include the use of the following guided tissue regeneration techniques: use of both resorbable and non-resorbable membranes and scaffolds, autologous grafts, allografts, xenografts, hydrogel and fibrin gels, cellular injections, cellular sheets, and various other biomimicking bone substitute materials.
Tissue Regeneration Techniques
There are four main types of tissue grafts typically found in regenerative medicine studies using MSCs: autologous grafts, allografts, xenografts, and synthetic grafts [48]. Autologous grafts are obtained from an individual somewhere in the body and then used as a treatment to that same individual at the site of regeneration even if it differs from where the cells were obtained. In an allogenic transplant, the cells are obtained from a donor and then transplanted to the recipient at the site of regeneration. Xenografts are transplants from a donor that is a different species than the recipient. Various other synthetic grafts exist that function to mimic the native tissue and aid in regeneration and allow the transplant recipient to avoid an autologous graft. The synthetic materials are based on cells obtained from BM-MSCs and are able to induce differentiation.
Cellular grafts can either be placed surgically or injected directly to the site of regeneration which does not require surgery. When the cell graft is injected, there is no structure accompanying the transplant, the cells are simply injected into the regeneration site and allowed to proliferate and integrate with the native tissue. A fibrin gel like material or a platelet rich plasma gel is often used in the injection to provide some medium for the cells during transplantation [49,50].
If the cells must be placed through surgical means, there is often a scaffold used as a transport medium to aid in transplanting the cells to the specific site of regeneration. Additionally, there is often a membrane placed between the tissue graft and the gingiva. The membrane alone does not result in tissue regeneration but instead functions to prevent the gingiva from growing towards the site of regeneration [51]. The membrane is not able to recruit surrounding stem cells or signaling molecules to induce differentiation. The function of an optimal scaffold is to provide a structure at the site of regeneration with enough initial strength to give space for the tissue regeneration to take place [52]. The scaffold should aid in osteogenesis, and eventually decompose and become integrated with the native tissue. The scaffold should not trigger an immune or inflammatory response. The following characteristics should be considered when determining which scaffold to use in a procedure: tendency to facilitate proliferation and differentiation of cells, allowance of cell migration, capability to adhere to cells, and ability to integrate into the native tissue structure. Various materials, both natural and synthetic, have been found to function well as cellular scaffolds. Some natural materials include collagen, elastin, fibrin, silk, chitosan, and glycosaminoglycans [53]. Also, there are different surfaces or textures of scaffolds created such as porous scaffolds and scaffolds with a gel consistency. The hydrogel scaffolds have been found to integrate well with native tissue and provide similar mechanical support mimicking that of the native tissues [54,55].
Cellular Sheet Alternative to Scaffolds
There exists a non-scaffold alternative option for transport of the cells: cellular sheets. Cellular sheets negate the need for a scaffold because the cells themselves form a macrostructure that functions like a scaffold. The cellular sheet imitates the extracellular matrix [56]. The cells are held together by different proteins on their membranes and are able to function with any defect anatomy to create the desired anatomy.
Growth Factors to Aid in Tissue Regeneration
Along with the scaffold providing structure for tissue regeneration, various growth factors can promote tissue generation by expediting the healing process at the site of regeneration. Whitman and Marx were the first to investigate platelet-rich plasma (PRP), a platelet gel that contains various growth factors to aid in tissue regeneration [57,58]. They determined it to aid the wound healing specifically by providing a feedback response signal based on inflammation levels at the site of regeneration [59-62]. Later, a type of PRP known as platelet rich in growth factors (PRGF) was developed which contained more growth factors than PRP and required less blood for use [63]. A major disadvantage to PRGF is that after processing, the PRGF was found to clot very quickly making clinical surgical use of PRGF difficult [63]. A different platelet gel was then developed called platelet rich fibrin (PRF) that contained various growth factors and unlike PRP and PRGF, contained a structure of elastic fibrin [64]. In 2013, Marrelli et al. [64] investigated the application of PRF into the surgical sites of immediately placed implants. They observed the complete filling of the peri-implant tissues and significant bone formation at the implant site as well [64].
MSCs in Treatment of Periodontal Disease
Chronic periodontal disease is characterized by severe inflammation of the tissue structure that surrounds the tooth and often results in both tissue and alveolar bone loss which can consequently lead to tooth loss. Nyman et al. were pioneers in regenerative medicine when they invented the first periodontal tissue regeneration in the field of dentistry in 1982 [65]. Conventional treatments of periodontal disease include various means of guided tissue regeneration procedures including but not limited to the utilising of natural or synthetic bone grafts, addition of various growth factors, and barrier membranes [66]. The goal of these methods in clinical application is to control inflammation while providing a healthy environment for the formation of tissues with similar structural and functional characteristics as the native tissues at that site. However, these conventional treatments of periodontal disease using guided tissue regeneration alone are not able to fully regenerate all of the necessary periodontal supporting tissues to be considered full periodontal regeneration [66]. The general goal of periodontal regeneration is to fully restore the lost or damaged PDL-cementum-alveolar bone complex. This in itself poses challenges because there are various types of tissues involved in the complex all of which must be regenerated in order for the periodontium to be considered fully regenerated [67]. Stem cells, specifically MSCs, have been and continue to be considered as an allogeneic cell-based method for treatment of periodontal disease, specifically based on the immunosuppressive effects they could have in an inflamed microenvironment [68,69]. MSCs must differentiate into odontoblasts, cementoblasts, and fibroblasts in order to fully regenerate the periodontium complex.
PDLSCs in Treatment of Periodontal Disease
PDLSCs possess the cellular capability to create an immune response explaining their consideration for treatment of advanced inflammatory periodontal disease [70,71]. PDLSCs were first identified in 2004 when they were derived from adult third molars [72]. PDLSCs demonstrate great proliferation and have differentiated into cells of the PDL, alveolar bone, cementum, blood vessels, and peripheral nerves [73,74].
In 2020, Park et al. [75] examined the regeneration of the periodontal complex utilizing human derived PDLSCs in the form of an engineered cellular sheet. The cells obtained were induced to differentiate in vitro then were implanted into immunocompromised mice and allowed to regenerate. Mineral deposition and formation of collagenous fibers were observed suggesting the ability of the PDLSCs to regenerate both alveolar bone and PDL-like tissues; however cementum must be regenerated also for full periodontal regeneration. Further studies must be performed on the specific growth factors needed for optimal regeneration of all tissues of periodontal complex [75].
DFSCs in Treatment of Periodontal Disease
DFSCs possess the necessary MSCs surface markers to aid in development of teeth prior to eruption. During tooth formation, DFSCs function to develop PDL precursor cells, cementoblasts, and odontoblasts suggesting their possible inclusion in treatment of periodontitis [76]. Limited experimentation shows the ability of DFSCs to generate all tissue types present in the periodontal complex [68]. This was confirmed in 2012 when Guo et al. [77] observed the regeneration of PDL-cementum-alveolar bone complex by ex vivo transplanted DFSCs in immunocompromised mice.
GMSCs in Treatment of Periodontal Disease
GMSCs were first identified in 2009 in the lamina propria layer of gingival tissue [78]. Human gingiva plays an important role in establishing teeth in the alveolar bone even though gingival tissue is not directly a component of the periodontal complex. These cells are to be considered for treatment of advanced periodontal disease specifically due to the minimally invasive means through which they may be accessed. In 2013, GMSCs were used in treatment of induced furcation defects in beagle dogs [79]. Yu et al. [79] observed that the GMSCs differentiated into osteoblasts, cementoblasts, and PDL fibroblasts and enhanced the regeneration of alveolar bone, cementum, and PDL in the dogs [79]. Similar findings were represented by Fawzy et al. [48] in 2016 confirming GMSCs as a source of MSCs which aid in regeneration of periodontal tissue.
Current research from Qiu et al. [80] compares the regeneration characteristics of GMSCs and PDLSCs [80]. Conditioned medium of both GMSCs and PDLSCs were compared in the molars of rats specifically at the site of periodontal defects. There was found to be no significant difference between the periodontal regeneration of conditioned medium from DPSCs and from GMSCs. Thus in addition to PDLSCs, GMSCs is confirmed as an alternate source of MSCs for periodontal tissue regeneration [80].
DPSCs in Treatment of Periodontal Disease
DPSCs have been shown to differentiate into chondrocytes, osteocytes, adipocytes, and neural cells [81,82]. However there exists support that DPSCs may not be the best MSCs to be used clinically for periodontal regeneration as indicated by Park et al. [68] in 2011, Hynes et al. [83] in 2012, and Bassir et al. [84] in 2016.
In 2017, Tomasello et al. [85] researched the ability of DPSCs and GMSCs to treat advanced periodontal disease specifically directing their investigation on the cells’ tendency to regenerate bone [85]. The DPSCs and GMSCs were taken from teeth already infected with periodontitis and then allowed to proliferate and differentiate in vitro. A control sample of cells was taken from healthy teeth and used for comparison. The cellular markers of the MSCs were not affected by the inflammation and the MSCs were still observed to have osteogenic capabilities in vitro. Tomasello et al. [85] also found that the periodontally infected DPSCs and GMSCs showed greater proliferation and osteogenic capacity than the control DPSCs and GMSCs which may be attributed to the proinflammatory cytokines present [85]. Tomasello et al., conclude their findings suggest the clinical application of autologous cell-based tissue engineering seems promising in the near future.
In 2011, Park et al. [68] researched the effectiveness of three types of dental tissue-derived adult stem cells to treat induced advanced periodontal disease specifically directing their attention to the condition of the apical region of the tooth root [68]. Ten-month-old beagle dogs served as both the source of stem cells and the test subjects for periodontal regeneration. The study aimed to mimic human advanced periodontitis and the subsequent treatment using autologous dental stem cell transplantations using the immature dog molars. The stem cells chosen were PDLSCs, DPSCs, and DFSCs. This study is significant because the induced periodontitis, including induced inflammation and attachment loss, in the canines serves as an more accurate representation of human periodontitis compared to previous in vitro studies or studies on smaller periodontal defect size as is the case when smaller animals participate in the study. PDLSCs were found to be the most effective at generating periodontal tissues when evaluated histologically, upon morphological inspection, and regarding clinical application [68]. Contrastingly, DPSCs were not observed to be capable of regenerating a cementum-like structure. The PDLSCs showed the best success in quality and quantity of tissue generated, clonogenic capability, proliferation, and calcium deposition in bone formation. Park et al. [68] call for further testing regarding the specific cytokines of MSCs that function to most successfully regenerate diseased periodontal tissue [68]. Thus, based on current research, it can be concluded that at the present time DPSCs are not the most suitable MSCs to be considered for periodontal tissue regeneration.
SCAP in Treatment of Periodontal Disease
SCAP were first identified in human immature permanent teeth in 2006[86]. SCAP demonstrate great proliferation, self-renewal, and differentiation capacity in various cell lineages. Li et al. [87] investigate the effect of SCAP on periodontal tissue regeneration in swine [87]. SCAP improved the periodontally infected tissue after the cells were transplanted to the site of periodontal disease via injection making them a promising candidate for human application in treatment of advanced periodontal disease.
SHED in Treatment of Periodontal Disease
SHED were first identified in 2003 when they were obtained from human dental pulp of exfoliated deciduous teeth [20]. SHED demonstrate great proliferation and have been proven to regenerate bone and dentin-like tissues and contribute to the initiation of bone regeneration. They also possess certain immunomodulatory functions explaining their consideration for treatment of advanced inflammatory periodontal disease [88]. In 2018 Gao et al. [88] observed the regeneration of periodontal tissues in rats in response to treatment of induced periodontitis with multidose transplantation of SHED [88]. Regeneration attributed to SHED was observed in the form of decreased inflammation, new attachments of the PDL, and increased alveolar bone volume. These findings suggest that SHED may positively contribute to periodontal regeneration and a decrease of the associated inflammation.
DSSCs in Treatment of Periodontal Disease
DSSCs are obtained from the dental socket following tooth extraction. In 2014, Nakajima et al. [44] experimented with DSSCs in the treatment in a periodontal defect model. They found the DSSCs demonstrate great proliferation and differentiate into osteocytes, adipocytes, and chondrocytes. The DSSCs when combined with β-TCP/PGA were shown to contribute to bone formation and form connective tissue like structures to that regenerated bone. When transplanted into the defected area, the DSSCs regenerated cementum-like and PDL-like tissues in addition to the alveolar bone [44].
In addition to researching the most effective source of stem cells for treatment of advanced periodontal disease, current research focuses on the ideal means of transplanting the stem cells to the periodontal complex as well as in what microenvironments the cells proliferate and differentiate most abundantly. In 2015, Cao et al. [89] investigated the effect of human growth factor on the regeneration of periodontal tissue using DPSCs as well as the advantages of using cellular sheets compared to dissociated cellular injections [89]. Periodontitis was induced in swine which were then treated with transplantations of DPSCs both with and without human growth factor. The DPSCs engineered sheets both with and without human growth factor showed greater tissue regeneration than either group of the injected cells. Cao et al. [89] recognize, however, that the engineered cellular sheets must be placed through surgical means while surgery is not required with the injections of DPSCs [89]. Additionally, it was found that the addition of the human growth factor to the DPSCs engineered sheets significantly increased the regeneration capacity of the transplanted cells. These results were later confirmed in a similar study by Hu et al. in 2016 [90].
In conclusion, research shows that the different sources of stem cells described above play varying roles in the regeneration of periodontal tissue in treatment of periodontal disease suggesting they are an innovative approach in the realm of personalized medicine to treat periodontal disease. The stem cells continue to be considered for treatment due to their general ability to differentiate into multiple lineages, proliferate, and maintain their stemness in an immunocompromised and inflamed environment. When considering the research discussed, the delivery of the MSCs to the periodontal disease site often resulted in regeneration of one or more of the tissues that comprise the periodontal complex or generated precursor cells that functioned to aid in inducing tissue formation. A major limitation to current research is that the majority of the studies are carried out using animal models. Due to the anatomical and oral bacterial differences between animal models and humans, it is difficult to recreate or mimic the correct microenvironment that is present in a human mouth. Human clinical trials are needed in multitudes before application of MSCs based treatment may become common in clinical practice. Additionally, future research should investigate and determine the adequate extracellular matrix scaffold, cellular sheets paired with bone graft, and other various complementary growth factors that help to optimize the function of the MSCs during transplantation. In general, the current application of MSCs in treatment of advanced periodontal disease while biologically possible, presently remains clinically unpredictable.
MSCs in Reconstruction of Craniofacial Defects
Possible explanations for a significant craniofacial bone defect are loss of bone due to the surgical extraction of a tooth and altered anatomy due to craniofacial trauma or genetic developmental skeletal conditions. These along with other various explanations for bone loss could result in the loss of ability to chew and functional tooth structure thus affecting overall health and appearance of the individual. MSCs are currently being explored as an option for treatment of craniofacial bone defects because of their ability to differentiate into both chondrocytes and osteoblasts. This differentiation potential suggests they may have a positive contribution to bone regeneration in craniofacial structures with bone defects. Thus, new and improving cellular-based bone regeneration therapies have much attention in the field of oral and craniofacial surgery. Tissue engineering and the application of MSCs is considered a state-of-the-art treatment option for bone regeneration [91]. Stutz et al. [92] researched the effectiveness of BM-MSCs in regeneration of the maxillary bone. Human BM-MSCs were transplanted to mice maxillary bone injured sites and induced to proliferate. Maxillary bone regeneration attributed to the transplanted cells was observed 90-150 days after transplantation. The injured site showed great osteogenesis upon histological analysis.
Specifically considering BM-MSCs from the alveolar bone, Liu et al. [93] compared the osteogenic capacity and differentiation potentials of human ABMSCs and BM-MSCs from the iliac bone both in vitro and in vivo [93]. Upon histological comparison in vitro, no apparent histological differences were observed. However, ABMSCs were found to have greater osteogenic differentiation capacity, showed greater mineral deposition, and showed more osteogenic marker genes when compared to BM-MSCs from the iliac crest. The stem cells were transferred via MSCs sheets to rabbit calvarial bone defects and allowed to proliferate and differentiate in vivo. ABMSCs regenerated bone that was observed to be both greater in volume and density, specifically trabecular thickness, compared to the BM-MSCs from the iliac bone [93]. Liu et al. [93] concluded that their finding suggest the use of ABMSCs to be considered for further testing working towards including this cellular therapy in a clinical setting [93].
Treatment of Bone Defects Related to Surgical Placement of Dental Implants
Placement of dental implants to restore function and appearance of the alveolar ridge is becoming more popular and accessible, however certain requirements exist regarding the anatomy or bone structure necessary to successfully place a dental implant and ensure it is maintained throughout the remainder of the individual’s life without failure. Alveolar bone augmentation and thickening is often needed as a step of the dental implant placement procedure. Sometimes a sinus augmentation is needed if there is alack in depth of bone and there is risk of sinus perforation during implant placement. Also, cellular scaffolds are often able to provide structure for bone regeneration where a certain amount of bone volume is necessary for placement of a dental implant. Current research regarding bone formation after surgical placement of dental implants focuses on the osseointegration of the implant, the surface or composition of the implant itself and how that may affect osseointegration, the crestal bone levels, and the regenerated bone density at the site of implantation. In 2009, McAllister et al. [96] performed a histologic evaluation of the efficacy of the use of a MSCs allograft in a sinus augmentation procedure. The cell populations were obtained from cadavers and cryopreserved. The cryopreserved cells are tested to determine if their MSCs characteristics and cellular markers still remain after cryopreservation. Although they obtained results suggesting the positive contribution of BM-MSCs.
In 2020, Piglionico et al. [94] investigated the effect of DPSCs on the osseointegration of implant in an in vitro implant model study. Both classical titanium and porous titanium implants were investigated with DPSCs. Testing was performed to observe the DPSCs adhesion capabilities to each implant surface, their differentiation capacity, and ability to proliferate. Observed significant delayed differentiation of the DPSCs on the porous titanium implants than was observed on the classical titanium implants. This is attributed to the DPSCs need to solely proliferate until they fully cover the porous implant before they begin differentiating. However, once differentiation was observed, the DPSCs showed greater proliferation and calcium deposition on the porous titanium implants [94]. These findings suggest the porous titanium implants along with the incorporation of DPSCs may incorporate into the native bone and allow greater osseointegration of the implant than the classical titanium implants would allow.
In 2021, Choi et al. [95] researched the impact of ABMSCs on the osseointegration of dental implants that have undergone different surface treatments [95]. The study was performed on rabbits using mini implants as an in vivo human implant model. The bone formation seen resulting from the integration of ABMSCs in implantation was statistically more than the implants without ABMSCs. These findings were confirmed radiographically and upon histological inspection suggesting the possible future clinical application of ABMSCs in placement of implants [95].
While both studies discussed above indicate positive results associated with the incorporation of either DPSCs or ABMSCs, there are limitations with each that must be acknowledged. The first study performed by Piglionico et al. [94] is an in vitro, and the second performed by Choi et al. [95] is an in vivo animal study. Application of these findings and research to human clinical testing must occur before the use of stem cells in dental implant therapy may become part of conventional treatment.
Treatment of Bone Defects Associated with Third Molar Extraction Sites
There are many different diagnoses to account for the reason a tooth must be extracted. In the case of third molars, they may be impacted, become infected if they partially erupt, or may grow and develop in a way that puts the stability of the second molars in question thus requiring their removal. Regarding all teeth not just the third molars, they could require extraction due to some traumatic injury that injures some part of the tooth structure. They may also be extracted as a part of orthodontic treatment if there are supernumerary teeth or if there is found to be overcrowding in the mouth. Additionally, tooth decay, disease, or abscessing at the tooth root may all require the extraction teeth. After a tooth is extracted, a tooth socket is left behind in the bone structure where the tooth once was. The cavity in the bone that is left behind is known as a bone defect, and treatment of this bone defect with MSCs may result in an expedited healing process or reducing the bone loss due to resorption.
In 2009, d’Aquinoet al. [96] investigated using DPSCs to repair bone defects left in individuals as a result of their third molars being extracted [96]. Specifically, the individuals included in the study expressed alveolar bone resorption bilaterally, and the defect created had no walls making unprompted bone generation unlikely and instead often induces loss of the second molars. Following extraction of the third molars, DPSCs were obtained from the molars and allowed to proliferate. The cells were then transplanted to the empty socket. Three months post treatment, the alveolar ridge was found to be restored vertically and periodontal restoration was observed along the second molars [96]. Histological and radiographic analysis showed full regeneration of the bone at the extraction site one year after treatment [96]. These findings suggest DPSCs aid in the bone regeneration of alveolar bone defects created by the extraction of third molars. However, in 2018, Barbier et al. [97] obtained results could not support the use of DPSCs in treatment of alveolar bone defects created by the extraction of third molars. In their study, DPSCs were transplanted to the extraction site of impacted third molars, specifically. Upon analysis of bone density and radiographic inspection there was no significant result to indicate that DPSCS reduced bone resorption [97].
Treatment of Temporomandibular Joint Osteoarthritis
There is limited research investigating the use of MSCs in treatment of temporomandibular joint (TMJ) disorders, but some research exists investigating the use of mesenchymal stem cell derived exosomes in treatment of TMJ disorders [98-101]. In 2020, Lee et al. [98] investigated the effectiveness of utilizing stem cell derived exosomes in tissue repair and tissue regeneration specifically to repair lost cartilage as they possess similar regeneration capabilities as the MSCs themselves [98]. The exosomes were investigated based on the immunomodulatory and anti-inflammatory effects that they possess similar to MSCs. Condylar cartilage poses low self-regeneration capacity thus, stem cells from oral sources should be considered and further analyzed for treatment of temporomandibular joint diseases.
Summary
The investigation of the use of orally derived stem cells has been on the forefront of research in the field of oral surgery due to the discovery of different sources of adult stem cells in oral tissues and their ease of accessibility. Progress is being made through research and the continued consideration of MSCs. These cell-based therapies discussed seem to be a promising approach for restoring various dental and maxillofacial tissues. Regardless of cell source, cell-based transplants seem to be superior to cell-free treatment options for oral tissue regeneration. In various clinical applications, MSCs have been used in combination with different scaffolds and growth factors to prove their benefit and ability to positively contribute to tissue regeneration, but the optimal combination of scaffold and growth factors must be determined. Most of the research to date uses solely in vitro experimentation and animal model studies. Thus, more human clinical trials should be conducted successfully to evaluate the efficacy of these cell-based therapies and increase the clinical predictability of these methods. The cost-effectiveness also should be taken into consideration. Current research indicates that cell-based regenerative therapy using dental derived stem cells is a promising tool for the treatment of various diseases in the future. However, the remaining obstacles must be overcome before stem cell use becomes a part of conventional treatment.
References
- Petersen PE. (2009) Global policy for improvement of oral health in the 21st century-implications to oral health research of World Health Assembly 2007, World Health Organization. Community Dent Oral Epidemio. 37(1):1-8.
- Paz AG, Maghaireh H, Mangano FG. (2018) Stem Cells in Dentistry: Types of Intra- and Extraoral Tissue-Derived Stem Cells and Clinical Applications. Stem Cells Int. 2018.
- Ohkoshi S, Hirono H, Nakahara T, Ishikawa H. (2018) Dental pulp cell bank as a possible future source of individual hepatocytes. World J Hepatol. 10(10):702-7.
- Caplan AI. (1991) Mesenchymal stem cells. J Orthop Res. 9(5):641-50.
- Mao JJ. (2008) Stem cells and the future of dental care. N Y State Dent J. 74(2):20-4.
- Ullah I, Subbarao RB, Rho GJ. (2015) Human mesenchymal stem cells- current trends and future prospective. Biosci Rep. 35(2).
- Arora V, Arora P, Munshi AK. (2009) Banking stem cells from human exfoliated deciduous teeth (SHED): saving for the future. J Clin Pediatr Dent. 33(4):289-94.
- Bianco P, Robey PG, Simmons PJ. (2008) Mesenchymal Stem Cells: Revisiting History, Concepts, and Assays. Cell Stem Cell. 2(4):313-9.
- Ab Kadir R, Zainal Ariffin SH, Megat Abdul Wahab R, Kermani S, et al. (2012) Characterization of mononucleated human peripheral blood cells. Sci World J. 1:843843.
- Sakata N, Yoshimatsu G, Kodama S. (2018) The Spleen as an Optimal Site for Islet Transplantation and a Source of Mesenchymal Stem Cells. Int J Mol Sci. 19(5).
- Imamura H, Adachi T, Kin T, Ono S, Sakai Y, et al. (2018) An engineered cell sheet composed of human islets and human fibroblast, bone marrow-derived mesenchymal stem cells, or adipose-derived mesenchymal stem cells: An in vitro comparison study. Islets. 10(3):e1445948.
- Mutlu L, Hufnagel D, Taylor HS. (2015) The endometrium as a source of mesenchymal stem cells for regenerative medicine. Biol Reprod. 92(6):138.
- Ding D, Chang Y, Shyu W, Lin S. (2015) Human umbilical cord mesenchymal stem cells: a new era for stem cell therapy. Cell Transplant. 24(3):339-47.
- Miyajima A, Tanaka M, Itoh T. (2014) Stem/progenitor cells in liver development, homeostasis, regeneration, and reprogramming. Cell Stem Cell. 14(5):561-74.
- Harvanova D, Tothova T, Sarissky M, Amrichova J, Rosocha J. (2011) Isolation and characterization of synovial mesenchymal stem cells. Folia Biol (Praha). 57(3):119-24.
- Williams AR, Hare JM. (2011) Mesenchymal stem cells: biology, pathophysiology, translational findings, and therapeutic implications for cardiac disease. Circ Res. 109(8):923-40.
- da Silva Meirelles L, Chagastelles PC, Nardi NB. (2006) Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 119(Pt 11):2204-13.
- Egusa H, Sonoyama W, Nishimura M, Atsuta I, Akiyama K. (2012) Stem cells in dentistry-part I: stem cell sources. J Prosthodont Res. 56(3):151-65.
- De Bari C, Dell'Accio F, Tylzanowski P, Luyten FP. (2001) Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 44(8):1928-42.
- Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, et al. (2003) SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci USA. 100(10):5807-12.
- Pierdomenico L, Bonsi L, Calvitti M, Rondelli D, Arpinati M, et al. (2005) Multipotent mesenchymal stem cells with immunosuppressive activity can be easily isolated from dental pulp. Transplant. 80(6):836-42.
- Nakashima M, Iohara K, Murakami M, Nakamura H, Sato Y, et al. (2017) Pulp regeneration by transplantation of dental pulp stem cells in pulpitis: a pilot clinical study. Stem Cell Res Ther. 8.
- Nakashima M. (2005) Bone morphogenetic proteins in dentin regeneration for potential use in endodontic therapy. Cytokine Growth Factor Rev. 16(3):369-76.
- Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. (2000) Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci USA. 97(25):13625-30.
- Har A, Park JC. (2015) Dental Stem Cells and Their Applications. Chin J Dent Res. 18(4):207-12.
- Hollands P, Aboyeji D, Orcharton M. (2018) Dental pulp stem cells in regenerative medicine. Br Dent J. 224(9):747-50.
- Tsutsui TW. (2020) Dental Pulp Stem Cells: Advances to Applications. Stem Cells Cloning. 13:33-42.
- Taguchi T, Yanagi Y, Yoshimaru K, Zhang X, Matsuura T, et al. (2019) Regenerative medicine using stem cells from human exfoliated deciduous teeth (SHED): a promising new treatment in pediatric surgery. Surg Today. 49(4):316-22.
- Seo B, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, et al. (2004) Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 364(9429):149-55.
- Sharpe PT. (2016) Dental mesenchymal stem cells. Develop. 143(13):2273-80.
- Zakrzewski W, Dobrzynski M, Szymonowicz M, Rybak Z. (2019) Stem cells: past, present, and future. Stem Cell Res Ther. 10(1):68.
- Morsczeck C, Gotz W, Schierholz J, Zeilhofer F, Kuhn U, et al. (2005) Isolation of precursor cells (PCs) from human dental follicle of wisdom teeth. Matrix Biol. 24(2):155-65.
- Dave JR, Tomar GB. (2018) Dental Tissue-Derived Mesenchymal Stem Cells: Applications in Tissue Engineering. Crit Rev Biomed Eng. 46(5):429-68.
- Tian Y, Bai D, Guo W, Li J, Zeng J, Yang L, et al. (2015) Comparison of human dental follicle cells and human periodontal ligament cells for dentin tissue regeneration. Regen Med. 10(4):461-79.
- Liu J, Yu F, Sun Y, Jiang B, Zhang W, et al. (2015) Concise reviews: Characteristics and potential applications of human dental tissue-derived mesenchymal stem cells. Stem Cells. 33(3):627-38.
- Ikeda E, Yagi K, Kojima M, Yagyuu T, Ohshima A, et al. (2008) Multipotent cells from the human third molar: feasibility of cell-based therapy for liver disease. Differentiation. 76(5):495-505.
- Zhang Q, Shi S, Liu Y, Uyanne J, Shi Y, et al. (2009) Mesenchymal stem cells derived from human gingiva are capable of immunomodulatory functions and ameliorate inflammation-related tissue destruction in experimental colitis. J Immunol. 183(12):7787-98.
- Matsubara T, Suardita K, Ishii M, Sugiyama M, Igarashi A, et al. (2005) Alveolar bone marrow as a cell source for regenerative medicine: differences between alveolar and iliac bone marrow stromal cells. J Bone Miner Res. 20(3):399-409.
- Cao C, Tarle S, Kaigler D. (2020) Characterization of the immunomodulatory properties of alveolar bone-derived mesenchymal stem cells. Stem Cell Res Ther. 11(1):102.
- Rai S, Kaur M, Kaur S. (2013) Applications of Stem Cells in Interdisciplinary Dentistry and Beyond: An Overview. Ann Med Health Sci Res. 3(2):245-54.
- Tamaki Y, Nakahara T, Ishikawa H, Sato S. (2013) In vitro analysis of mesenchymal stem cells derived from human teeth and bone marrow. Odontolog. 101(2):121-32.
- Sonoyama W, Liu Y, Fang D, Yamaza T, Seo B, et al. (2006) Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS ONE. 1:e79.
- Kang J, Fan W, Deng Q, He H, Huang F. (2019) Stem Cells from the Apical Papilla: A Promising Source for Stem Cell-Based Therapy. Biomed Res Int. 2019:6104738.
- Nakajima R, Ono M, Hara ES, Oida Y, Shinkawa S, et al. (2014) Mesenchymal stem/progenitor cell isolation from tooth extraction sockets. J Dent Res. 93(11):1133-40.
- Paz AG, Maghaireh H, Mangano FG. (2018) Stem Cells in Dentistry: Types of Intra- and Extraoral Tissue-Derived Stem Cells and Clinical Applications. Stem Cells Int. 2018:4313610.
- Chatzivasileiou K, Kriebel K, Steinhoff G, Kreikemeyer B, Lang H. (2015) Do oral bacteria alter the regenerative potential of stem cells? A concise review. J Cell Mol Med. 19(9):2067-74.
- Rodriguez-Lozano F, Insausti C, Iniesta F, Blanquer M, Ramirez M, et al. (2012) Mesenchymal dental stem cells in regenerative dentistry. Med Oral Patol Oral Cir Bucal. 17(6):1062.
- Fawzy El-Sayed KM, Dorfer CE. (2016) Gingival Mesenchymal Stem/Progenitor Cells: A Unique Tissue Engineering Gem. Stem Cells Int. 2016:7154327.
- Ito K, Yamada Y, Nakamura S, Ueda M. (2011) Osteogenic potential of effective bone engineering using dental pulp stem cells, bone marrow stem cells, and periosteal cells for osseointegration of dental implants. Int J Oral Maxillofac Implants. 26(5):947-54.
- Yamada Y, Ueda M, Naiki T, Takahashi M, Hata K, et al. (2004) Autogenous injectable bone for regeneration with mesenchymal stem cells and platelet-rich plasma: tissue-engineered bone regeneration. Tissue Eng. 10(5-6):955-64.
- Lin L, Chen MY, Ricucci D, Rosenberg PA. (2010) Guided tissue regeneration in periapical surgery. J Endod. 36(4):618-25.
- Horst OV, Chavez MG, Jheon AH, Desai T, Klein OD. (2012) Stem Cell and Biomaterials Research in Dental Tissue Engineering and Regeneration. Dent Clin North Am. 56(3):495-520.
- Galler KM, D'Souza RN, Hartgerink JD, Schmalz G. (2011) Scaffolds for dental pulp tissue engineering. Adv Dent Res. 23(3):333-9.
- Smith EL, Kanczler JM, Gothard D, Roberts CA, Wells JA, et al. (2014) Evaluation of skeletal tissue repair, part 1: assessment of novel growth-factor-releasing hydrogels in an ex vivo chick femur defect model. Acta Biomater. 10(10):4186-96.
- Smith EL, Kanczler JM, Gothard D, Roberts CA, Wells JA, et al. (2014) Evaluation of skeletal tissue repair, part 2: enhancement of skeletal tissue repair through dual-growth-factor-releasing hydrogels within an ex vivo chick femur defect model. Acta Biomater. 10(10):4197-205.
- Hirose M, Kwon OH, Yamato M, Kikuchi A, Okano T. (2000) Creation of designed shape cell sheets that are noninvasively harvested and moved onto another surface. Biomacromolecules. 1(3):377-81.
- Marx RE, Carlson ER, Eichstaedt RM, Schimmele SR, Strauss JE, et al. (1998) Platelet-rich plasma: Growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 85(6):638-46.
- Whitman DH, Berry RL, Green DM. (1997) latelet gel: an autologous alternative to fibrin glue with applications in oral and maxillofacial surgery. J Oral Maxillofac Surg. 55(11):1294-9.
- Gibble JW, Ness PM. (1990) Fibrin glue: the perfect operative sealant? Transfusion. 30(8):741-7.
- Saltz R, Sierra D, Feldman D, Saltz MB, Dimick A, et al. (1991) Experimental and clinical applications of fibrin glue. Plast Reconstr Surg. 88(6):1005-17.
- Hotz G. (1991) Alveolar ridge augmentation with hydroxylapatite using fibrin sealant for fixation. Part I: An experimental study. Int J Oral Maxillofac Surg. 20(4):204-7.
- Hotz G. (1991) Alveolar ridge augmentation with hydroxylapatite using fibrin sealant for fixation. Part II: Clinical application. Int J Oral Maxillofac Surg. 20(4):208-13.
- Weibrich G, Kleis WKG, Hafner G, Hitzler WE, Wagner W. (2003) Comparison of platelet, leukocyte, and growth factor levels in point-of-care platelet-enriched plasma, prepared using a modified Curasan kit, with preparations received from a local blood bank. Clin Oral Implants Res. 14(3):357-62.
- Marrelli M, Tatullo M. (2003) Influence of PRF in the healing of bone and gingival tissues. Clinical and histological evaluations. Eur Rev Med Pharmacol Sci. 17(14):1958-62.
- Nyman S, Lindhe J, Karring T, Rylander H. (1982) New attachment following surgical treatment of human periodontal disease. J Clin Periodontol. 9(4):290-6.
- Mudda JA, Bajaj M. (2011) Stem cell therapy: A challenge to periodontist. Indian J Dent Res. 2(1):132.
- Polimeni G, Xiropaidis AV, Wikesjo UME. (2006) Biology and principles of periodontal wound healing/regeneration. Periodontol 2000. 41:30-47.
- Park J, Jeon SH, Choung P. (2011) Efficacy of Periodontal Stem Cell Transplantation in the Treatment of Advanced Periodontitis. Cell Transplant. 20(2):271-86.
- Zhou Y, Zheng L, Zhou X, Li J, Xu X. (2015) Dental Mesenchymal Stem Cells in Inflamed Microenvironment: Potentials and Challenges for Regeneration. Curr Stem Cell Res Ther. 10(5):412-21.
- Liu J, Chen B, Bao J, Zhang Y, Lei L, et al. (2019) Macrophage polarization in periodontal ligament stem cells enhanced periodontal regeneration. Stem Cell Res Ther. 10(1):320.
- Shin C, Kim M, Han J, Choi B, Hwang D, et al. (2017) Human periodontal ligament stem cells suppress T-cell proliferation via down-regulation of non-classical major histocompatibility complex-like glycoprotein CD1b on dendritic cells. J Periodontal Res. 52(1):135-46.
- Seo B, Miura M, Gronthos S, Bartold PM, Batouli S, et al. (2004) Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 364(9429):149-55.
- Martinez C, Smith PC, Rodriguez JP, Palma V. (2011) Sonic hedgehog stimulates proliferation of human periodontal ligament stem cells. J Dent Res. 90(4):483-8.
- Tomokiyo A, Yoshida S, Hamano S, Hasegawa D, Sugii H, et al. (2018) Detection, Characterization, and Clinical Application of Mesenchymal Stem Cells in Periodontal Ligament Tissue. Stem Cells Int. 2018:5450768.
- Park J, Park CH, Yi T, Kim S, Iwata T, et al. (2020) rhBMP-2 Pre-Treated Human Periodontal Ligament Stem Cell Sheets Regenerate a Mineralized Layer Mimicking Dental Cementum. Int J Mol Sci. 21(11):3767.
- Yang H, Li J, Hu Y, Sun J, Guo W, Li H, et al. (2019) Treated dentin matrix particles combined with dental follicle cell sheet stimulate periodontal regeneration. Dent Mater. 35(9):1238-53.
- Guo W, Chen L, Gong K, Ding B, Duan Y, et al. (2012) Heterogeneous dental follicle cells and the regeneration of complex periodontal tissues. Tissue Eng Part A. 18(5-6):459-70.
- Zhang Q, Shi S, Liu Y, Uyanne J, Shi Y, et al. (2009) Mesenchymal stem cells derived from human gingiva are capable of immunomodulatory functions and ameliorate inflammation-related tissue destruction in experimental colitis. J Immunol. 183(12):7787-98.
- Yu X, Ge S, Chen S, Xu Q, Zhang J, et al. (2013) Human gingiva-derived mesenchymal stromal cells contribute to periodontal regeneration in beagle dogs. Cells Tissues Organs. 198(6):428-37.
- Qiu J, Wang X, Zhou H, Zhang C, Wang Y, et al. (2020) Enhancement of periodontal tissue regeneration by conditioned media from gingiva-derived or periodontal ligament-derived mesenchymal stem cells: a comparative study in rats. Stem Cell Res Ther. 11.
- Fernandes TL, Cortez de SantAnna, Joao Paulo, Frisene I, Gazarini JP, Gomes Pinheiro CC, et al. (2020) Systematic Review of Human Dental Pulp Stem Cells for Cartilage Regeneration. Tissue Eng Part B Rev 26(1):1-12.
- Maioli M, Basoli V, Santaniello S, Cruciani S, Delitala AP, et al. (2016) Osteogenesis from Dental Pulp Derived Stem Cells: A Novel Conditioned Medium Including Melatonin within a Mixture of Hyaluronic, Butyric, and Retinoic Acids. Stem Cells Int. 2056416.
- Hynes K, Menicanin D, Gronthos S, Bartold PM. (2012) Clinical utility of stem cells for periodontal regeneration. Periodontol 2000. 59(1):203-27.
- Bassir SH, Wisitrasameewong W, Raanan J, Ghaffarigarakani S, Chung J, et al. (2016) Potential for Stem Cell-Based Periodontal Therapy. J Cell Physiol. 231(1):50-61.
- Tomasello L, Mauceri R, Coppola A, Pitrone M, Pizzo G, et al. (2017) Mesenchymal stem cells derived from inflamed dental pulpal and gingival tissue: a potential application for bone formation. Stem Cell Res Ther. 8.
- Sonoyama W, Liu Y, Yamaza T, Tuan RS, Wang S, et al. (2008) Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: a pilot study. J Endod. 34(2):166-71.
- Li G, Han N, Zhang X, Yang H, Cao Y, et al. (2018) Local Injection of Allogeneic Stem Cells from Apical Papilla Enhanced Periodontal Tissue Regeneration in Minipig Model of Periodontitis. Biomed Res Int. 2018:3960798.
- Gao X, Shen Z, Guan M, Huang Q, Chen L, et al. (2018) Immunomodulatory Role of Stem Cells from Human Exfoliated Deciduous Teeth on Periodontal Regeneration. Tissue Eng Part A. 24(17-18):1341-53.
- Cao Y, Liu Z, Xie Y, Hu J, Wang H, et al. (2015) Adenovirus-mediated transfer of hepatocyte growth factor gene to human dental pulp stem cells under good manufacturing practice improves their potential for periodontal regeneration in swine. Stem Cell Res Ther. 6:249.
- Hu J, Cao Y, Xie Y, Wang H, Fan Z, Wang J, et al. (2016) Periodontal regeneration in swine after cell injection and cell sheet transplantation of human dental pulp stem cells following good manufacturing practice. Stem Cell Res Ther. 7(1).
- Khojasteh A, Behnia H, Dashti SG, Stevens M. (2012) Current trends in mesenchymal stem cell application in bone augmentation: a review of the literature. J Oral Maxillofac Surg. 70(4):972-82.
- Stutz C, Strub M, Clauss F, Huck O, Schulz G, et al. (2020) A New Polycaprolactone-Based Biomembrane Functionalized with BMP-2 and Stem Cells Improves Maxillary Bone Regeneration. Nanomaterials (Basel). 10(9).
- Liu Y, Wang H, Dou H, Tian B, Li L, et al. (2020) Bone regeneration capacities of alveolar bone mesenchymal stem cells sheet in rabbit calvarial bone defect. J Tissue Eng. 11:2041731420930379.
- Piglionico S, Bousquet J, Fatima N, Renaud M, Collart-Dutilleul P, et al. (2020) Porous Tantalum vs. Titanium Implants: Enhanced Mineralized Matrix Formation after Stem Cells Proliferation and Differentiation. J Clin Med. 9(11).
- Choi H, Park K, Jung N, Shim J, Moon H, et al. (2021) In Vivo Study for Clinical Application of Dental Stem Cell Therapy Incorporated with Dental Titanium Implants. Materials (Basel). 14(2).
- d'Aquino R, De Rosa A, Lanza V, Tirino V, Laino L, et al. (2009) Human mandible bone defect repair by the grafting of dental pulp stem/progenitor cells and collagen sponge biocomplexes. Eur Cell Mater. 18:75-83.
- Barbier L, Ramos E, Mendiola J, Rodriguez O, Santamaria G, et al. (2018) Autologous dental pulp mesenchymal stem cells for inferior third molar post-extraction socket healing: A split-mouth randomised clinical trial. Med Oral Patol Oral Cir Bucal. 23(4):e469-77.
- Lee Y, Park H, Auh Q, Nah H, Lee JS, et al. (2020) Emerging Potential of Exosomes in Regenerative Medicine for Temporomandibular Joint Osteoarthritis. Int J Mol Sci. 21(4).
- Luo P, Jiang C, Ji P, Wang M, Xu J. (2018) (2019) Exosomes of stem cells from human exfoliated deciduous teeth as an anti-inflammatory agent in temporomandibular joint chondrocytes via miR-100-5p/mTOR. Stem Cell Res Ther. 10.
- Meijer GJ, de Bruijn JD, Koole R, van Blitterswijk CA. (2008) Cell based bone tissue engineering in jaw defects. Biomaterials. 29(21):3053-61.
- Cui D, Li H, Xu X, Ye L, Zhou X, et al. (2017) Mesenchymal Stem Cells for Cartilage Regeneration of TMJ Osteoarthritis. Stem Cells Int. 2017.

