Involvement of Reactive Oxygen Species (ROS) and Coagulation in Coronaviral Infection
Shihori Tanabe*
Senior Researcher, Division of Risk Assessment, Center for Biological Safety and Research, National Institute of Health Sciences, Japan
*Corresponding author: Shihori Tanabe, Senior Researcher, Division of Risk Assessment, Center for Biological Safety and Research, National Institute of Health Sciences, Japan
Citation: Tanabe S. (2021) Involvement of Reactive Oxygen Species (ROS) and Coagulation in Coronaviral Infection. Adv Clin Med Res. 2(2):1-4.
Received: September 29, 2021 | Published: October 11, 2021
Copyright© 2021 genesis pub by Tanabe S. CC BY-NC-ND 4.0 DEED. This is an open-access article distributedunder the terms of the Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International License.,This allows others distribute, remix, tweak, and build upon the work, even commercially, as long as they credit the authors for the original creation.
DOI: https://doi.org/10.52793/ACMR.2021.2(2)-21
Abstract
Coronaviral infection induces various molecular network pathways. Coronavirus pathogenesis pathway is involved in molecules in production of reactive oxygen species (ROS), oxidative stress responses and coagulation system. Several literatures have revealed the association of ROS and coagulation in infection of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). In this Editorial, the involvement of ROS and coagulation in coronavirus infectious disease (COVID)-19 pathogenesis is summarized.
Reactive Oxygen Species (ROS) in Coagulation System
ROS are oxygen-derived molecules that oxidize molecules or are converted into oxygen radicals [1]. ROS have dual-effects which are cell damaging or beneficial roles [1-3]. ROS generated by NOX2, a NADPH oxidase, in macrophage play an important role in killing of phagocytosed microorganisms [3]. The ROS accumulation cause mitochondrial dysfunction which leads to coagulopathy associated with inflammatory signaling pathways [4]. Polymorphonuclear leucocytes, commonly referred to as neutrophils, generate large amounts of ROS via the NADPH oxidase complex [5]. The ROS released from the polymorphonuclear leucocytes following trauma and haemorrhagic shock led to lung injury and coagulopathy [5]. Serpin family A member 1 (SERPINA1/alpha-1-antitrypsin), a serine protease inhibitor, inhibits coagulation factor 2a (thrombin) [6]. SERPINA1 is a member of low-density lipoprotein (LDL) and involved in ROS network [7]. ROS are required for release of granzyme B (GzmB), a cytotoxic lymphocyte protease, into the cytosol [8]. SERPINA1 is converted into a ROS-sensitive granzyme B (GzmB) inhibitor by replacing the P4-P3’ reactive center loop residues [8]. Thrombin activates NADPH oxidase and produces ROS, which leads to fibroblast proliferation [9]. Endothelial exposure of thrombin induces NOX-dependent superoxide superoxide anion and hydrogen peroxide [10,11].
How is reactive oxygen species (ROS) involved in coronavirus infection?
ROS is generated upon the infection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in coronavirus disease 2019 (COVID-19), and induces oxidative stress [12]. SARS-CoV-2 infection induces cytokine storm [13,14]. Cytokine storm includes ROS-induced oxidative stress and immune cell dysregulation. Glutathione S-transferase genes, which have functions in the elimination of ROS, involves the morbidity and mortality from COVID-19 [14]. The heme oxygenase-1 (HO-1) induction may be involved in the inflammation-induced coagulation in COVID-19 [14]. ROS quenching by vitamin C, E, b-carotene and polyphenols has been suggested in COVID-19 in the point of view of the nutrient, since oxidative stress causes inflammation [15]. Potential roles of omega-3 fatty acids accompanied by antioxidants have been suggested in the cytokine storm due to SARS-CoV-2 infection [16].
Coagulation and Coronavirus
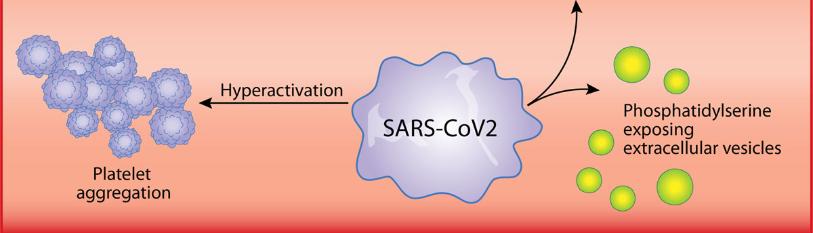
SARS-CoV-2 enters the blood stream and promotes coagulation cascade and induces blood clots [12]. SARS-CoV-2 upregulates complement and coagulation cascade in macaques [17]. Coagulation abnormalities and thrombosis are observed in SARS-CoV-2 infection [18]. COVID-19 patients have an increased risk of arterial and venous thrombosis, and elevated D-dimer levels are associated with the increased thrombosis and mortality [18]. The elevated D-dimer and fibrin degradation product levels are associated with poor prognosis in patients with SARS-CoV-2 pneumonia [19]. Cytokine storm and inflammation leads to the increases in D-dimer and poor prognosis of COVID-19 [20]. During early phase of SARS-CoV-2 infection, whereas coagulation test abnormalities are seen, they do not result in clinical bleeding [21]. Excess coagulation progresses disseminated intravascular coagulation (DIC) in COVID-19 patients at the later phase of the diseases [21]. Severe infections cause activation of coagulation, where coagulation itself is not clinically relevant, however robust coagulation leads to consumption of clotting factors and platelets and coagulation proteins, and DIC that is characterized by the thrombotic deposition in the microvasculature and increased bleeding tendency [22]. The activation of the coagulation system and the following DIC is observed in patients with severe leptospirosis as well [23]. Various physical properties of blood cells, such as lymphocyte stiffness, monocyte size, neutrophil size and deformability, and heterogeneity of erythrocyte deformation and size are altered in COVID-19 [24]. Oxygen delivery of erythrocyte might be affected by the changes in the physical properties of blood cells in COVID-19 [24]. COVID-19 pathogenesis involves renin-angiotensin system and bradykinin system, of which dysregulation causes hypokalemia, hyper-permeability, inflammation, hypotension, vasodilation, and pulmonary edema, as well [25]. The careful consideration in coagulopathies is needed to understand the coronavirus pathogenesis.
Conclusion
The involvement of ROS and coagulation system in coronavirus pathogenesis has been investigated in this article. Coagulation abnormalities and thrombosis are associated with severe condition in COVID-19, where ROS seem to have a role in coagulation cascade and cytokine storm. The mechanism in which ROS play a role on coronavirus infectious disease need to be carefully focused.
Acknowledgements
This work was supported by Japan Agency for Medical Research and Development (AMED), Grant Number JP21mk0101216, JSPS KAKENHI Grant Number 21K12133, and Ministry of Health, Labour, and Welfare (MHLW). The author would like to thank all collaborators.
References
- André-Lévigne D, Modarressi A, Pepper MS, Cuenod BP. Reactive Oxygen Species and NOX Enzymes Are Emerging as Key Players in Cutaneous Wound Repair. Int J Mol Sci. 18(10):2149.
- Beckman KB, BN Ames. (1998) The free radical theory of aging matures. Physiol Rev. 78(2):547-81.
- Bedard K, KH Krause. (2007) The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 87(1):245-313.
- Saleh J, Peyssonaux, Singh KK, Edeas M. (2020) Mitochondria and microbiota dysfunction in COVID-19 pathogenesis. Mitochondrion. 54:1-7.
- Barrett CD, et al., Blood clotting and traumatic injury with shock mediates complement-dependent neutrophil priming for extracellular ROS, ROS-dependent organ injury and coagulopathy. Clin Exp Immunol. 194(1):103-117.
- Cohen AB. (1973) Mechanism of action of alpha-1-antitrypsin. J Biol Chem. 248(20):7055-9.
- Lubrano V, S Balzan. (2020) Role of oxidative stress-related biomarkers in heart failure: galectin 3, α1-antitrypsin and LOX-1: new therapeutic perspective? Mol Cell Biochem. 464(1-2):143-152.
- Mangan MS, Bird HS, Kaiserman D, Matthews AY, Hitchen C, et al. ( 2016) A Novel Serpin Regulatory Mechanism: SerpinB9 is reversibly inhibited by vicinal disulfide bond formation in the reactive center loop. J Biol Chem. 291(7):3626-38.
- Zhou SY, Xiao W, Pan XJ, Zhu MX, Yang ZH, et al., Thrombin promotes human lung fibroblasts to proliferate via NADPH oxidase/reactive oxygen species/extracellular regulated kinase signaling pathway. Chin Med J (Engl). 123(17):2432-9.
- Pai WY, Lo WY, Hsu T, Peng CT, Wang Hj. (2017) Angiotensin-(1-7) Inhibits Thrombin-Induced Endothelial Phenotypic Changes and Reactive Oxygen Species Production via NADPH Oxidase 5 Downregulation. Front Physiol. 8:994.
- Holland JA, Meyer JW, Donnell RW, Johnson DK, Ziegler LM. (1998) Thrombin Stimulated Reactive Oxygen Species Production in Cultured Human Endothelial Cells. Endothelium. 6(2):113-121.
- Janardhan VV, Kalousek V. (2020) COVID-19 as a Blood Clotting Disorder Masquerading as a Respiratory Illness: A Cerebrovascular Perspective and Therapeutic Implications for Stroke Thrombectomy. J Neuroimaging. 30(5): p. 555-561.
- Frisoni P, Neri M, D’Errico S, Alfieri L, Bonuccelli D, et al. (2021) Cytokine storm and histopathological findings in 60 cases of COVID-19-related death: from viral load research to immunohistochemical quantification of major players IL-1β, IL-6, IL-15 and TNF-α. Forensic Sci Med Pathol. 31:1-15.
- Kaidashev I, Shlykova O, Izmailova O, Torubara O, Yushchenko Ya, et al. (2021) Host gene variability and SARS-CoV-2 infection: A review article. Heliyon. 7(8): e07863.
- Iddir M, Brito A, Dingeo G, Fernandez Del Campo SS, Samouda H, et al. (2020) Strengthening the Immune System and Reducing Inflammation and Oxidative Stress through Diet and Nutrition: Considerations during the COVID-19 Crisis. Nutrients. 12(6):1562.
- Rogero MM. (2020) Potential benefits and risks of omega-3 fatty acids supplementation to patients with COVID-19. Free Radic Biol Med. 156:190-99.
- Aid M, Sahay KB, Vidal SJ, Maliga Z, Bondoc S, et al. (2020) Vascular Disease and Thrombosis in SARS-CoV-2-Infected Rhesus Macaques. Cell. 183(5):1354-1366.e13.
- Mackman N, Antoniak S, Wolberg AS, Kasthuri R, Key NS. (2020) Coagulation Abnormalities and Thrombosis in Patients Infected With SARS-CoV-2 and Other Pandemic Viruses. Arteriosclerosis, thrombosis, and vascular biology. 40(9):2033-44.
- Tang N, Li D, Wang X, Sun Z. (2020) Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 18(4):844-47.
- de la Morena-Barrio, Perez CB, Minano A, de la Morena-Barrio B, Minano A, et al., (2021) Prognostic value of thrombin generation parameters in hospitalized COVID-19 patients. Sci Rep. 11(1):7792.
- Connors JM, JH Levy. (2020) COVID-19 and its implications for thrombosis and anticoagulation. Blood. 135(23):2033-40.
- Levi M. (2018) Pathogenesis and diagnosis of disseminated intravascular coagulation. Int J Lab Hematol . 40(S1):15-20.
- Wagenaar JFP, Goros MGA, Partiningrum DL, Isbandrio B, Hartskeerl RA, et al. (2010) Coagulation disorders in patients with severe leptospirosis are associated with severe bleeding and mortality. Trop Med Int Health. 15(2):152-59.
- Kubánková M, Hohberger B, Hoffmanns J, Julia Fürst ,Herrmann M. (2021) Physical phenotype of blood cells is altered in COVID-19. Biophysical J. 120(14):2838-47.
- Garvin MR, Alvarez C, Miller JI, Prates ET, Walker Am, et al. (2020) A mechanistic model and therapeutic interventions for COVID-19 involving a RAS-mediated bradykinin storm. eLife. 9:e59177.

