Idiopathic Thrombocytopenic Purpura in Tuberculosis
Marsela Shani* and Drita Jaka
Prison Health Hospital (Mother Teresa University Hospital) Tirana Albania
*Corresponding author: Marsela Shani, Prison Health Hospital (Mother Teresa University Hospital) Tirana Albania
Citation: Shani M, Jaka D. (2022) Idiopathic Thrombocytopenic Purpura in Tuberculosis. Adv Clin Med Res. 3(1):1-6.
Received: April 08, 2022 | Published: May 20, 2022
Copyright© 2022 genesis pub by Shani M, et al. CC BY-NC-ND 4.0 DEED. This is an open-access article distributedunder the terms of the Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International License.,This allows others distribute, remix, tweak, and build upon the work, even commercially, as long as they credit the authors for the original creation.
DOI: https://doi.org/10.52793/ACMR.2022.3(1)-27
Abstract
Tuberculosis (TB) is a contagious disease in both developing and developed countries. The incidence is increasing due to bacilli resistant to many drugs and the human immunodeficiency virus (HIV). Since 2012, India has the highest incidence of the disease at around 2.2 million cases, accounting for 26% of the global incidence according to World Health Organization statistics. A wide range of hematological manifestations is observed in TB, where thrombocytopenia is common in miliary TB and thrombocytosis in pulmonary TB. Immune thrombocytopenic purpura (ITP) is characterized by a low platelet count accompanied by the presence of autoantibodies. The association of immune thrombocytopenic purpura and tuberculosis is a rare condition. In the case presented, anti-tuberculosis therapy was effective for both tuberculosis and thrombocytopenia, suggesting a causal link between tuberculosis and immune thrombocytopenic purpura.
Purpose: Reporting a case of association of PTI and TBC.
Method: Clinical case study and correlation with literature.
Discussion: The pathophysiology of thrombocytopenia in tuberculosis remains unanswered. This is a rare condition, estimated to occur in less than 1% of tuberculosis cases. Mycobacterium tuberculosis can break down the antigen with platelets leading to the formation of antiplatelet antibodies. HLA-specific presentation of tuberculosis may also lead to the antiplatelet immune response in some patients.
Keywords
PTI; Thrombocytopenia; Mycobacterium tuberculosis; TBC; Antituberculous therapy
Introduction
Patient S.SH, male, age 64 years, born Korca (known endemic area for TBC) is referred by the district hematologist as suspect immune thrombocytopenia. The patient's complaints were related to fatigue, weakness, sweating, blood in the urine, blood through saliva and black stools, which the patient had been complaining about for almost 4-5 days.
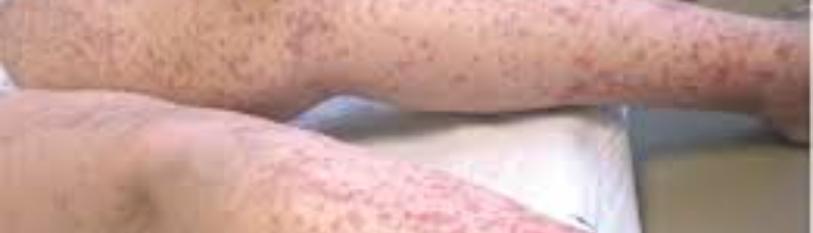
Objective examination: Pale on the skin and mucous membranes with obvious signs of cutaneous hemorrhage in the form of diffuse ecchymosis and petechiae, dense, widespread throughout the body but more evident in the upper and lower bilateral extremities as well as at the level of the right hemithorax and abdomen. Patient with signs of urinary tract hemorrhage (evident in the catheter bag) as well as bleeding gums all the time.
Clear conscience, rhythmic cardiac tone with frequency 85bpm, TA = 100 / 80mmHg, attenuated respiration more expressed on the right side, Sat O₂ 96% under room air, soft abdomen. Treatable not dolent on palpation abdomen, without muscle protection, hepar and lien below the costal arch, no peripheral palpable lymphadenopathy. Anamnesis Vitae: Patient Known as Diabetes Mellitus type 2 under treatment with oral antidiabetics, HTA under treatment with antihypertensives.
Family history: Nothing to note.
Examinations showed Hemograms RBC 3.41x10⁶, Hb 9.0gr / dl, Hct 26.9%, MCV 79.0fL, RDË 14.6%, WBC 8.2K / uL, PLT 2K / uL (norm 150-400). Thrombocytes in the blade: rare in preparations.
Bone marrow aspiration: Promyelocytes 1%, Neutrophil myelocytes 2%, Neutrophil metamyelocytes 8%, Neutrophil sticks 22%, Segments 25%, Lymphocytes 13%, Monocytes 7%, Polychromatophilic normoblasts 12%, Oxyphilic normoblasts 8%, Plasma cell 2%. rich in material, M / E ratio = 2.9 / 1. Megakaryocyte apparatus present with a small platelet burst.
Leukocyte immunophenotype: Bone marrow material. Through leukocyte immunophenotyping was observed: About 1% of cells with the markers CD34 +, CD117 +, HLA-DR +, CD13 +, CD33 + probably myeloblasts.
-Lymphoid population makes up about 6% of all cells which were studied for the above markers.
-Granular series which makes up about 79% of all cells parasites with normal granularity.
-Monocyte population makes up about 4% of all cells.
-The red series makes up about 11% of all cells.
Coagulation panel: PT 72%, INR 1.24, aPTT 23.0sec, Fibrinogen 471mg / dL, D-dimer 0.82µg / mL.
Biochemical balance: Glucose 157mg / dL, Urea 124.2mg / dL, Creatinine 1.18mg / dL, Direct bilirubin 0.33mg / dL, ALT 11UI / L, AST 16U / L, GGT 28U / L, APL 50U / L, LDH 259U / L, CK 132U / L, Calcium 8.8mg / dL, CRP 1.46mg / dL.
Immune panel: HBsAg0.30S / CO (<0.99 neg) Anti-HCV 0.88S / CO (<0.99 neg), Anti-HBs 0.09IU / L (<9.99 neg), AntiHBc 0.17S / CO (<1 neg), HIV 1 & 2 Ab, RDT negative, ANA <160 (negative), Anti ds DNA 8.4IU / mL (<35, neg) ENA 0.10U / mL (<0.8 neg), MPO 2.9U / mL (<10 neg). CEA 0.8 (<10 neg), AFP 3.8 (<8.8 neg), CA 19-9 10.4 (<37 neg), CA 15-3 9.9U / mL (<31.3 neg), Total PSA 0.43ng / mL, Ferritine 130.70 ng / mL.
Lung CT: Trachea, free main bronchi. Sinister pleural thickening with posterobasal effusion with DIP 16mm. Multiple pseudonodular consolidations in both lungs.
Protein electrophoresis: Albumin 58.5%, alpha 1-globulinate 5.3%, alpha 2-globulinate 13%, beta 1-globulinate 5.5%, beta 2-globulinate 4.3%, gammaglobulinate 13.4%, Ratio A / G 1.41.
After completion with the necessary examination panels the patient was placed under corticosteroid therapy with a maximum dose of 2 mg / kg body weight.
On 08/03/2022 the patient is consulted by a pulmonologist who requires equipment with Sput-BK 3 samples which Geneexpert sputum (Positive) showed the presence of DNA for MTB positive. The patient was placed from 11/3/2022 under the anti-tuberculosis regime with:
-Rifampicine / Isoniazid 150/75 mg 3tb per day oral.
-Pyrazinamide 500 mg 2tb per day oral.
-Gambutol 400 mg 2 tb per day oral.
-Multivitamine 3x1 tb per day oral (Figure 1).
Figure 1: Course of platelet counts before and after the start of antituberculous therapy.
The graph below shows the correlation between the doses of cortisone used by the patient and the value of PLT. The patient from 11/03/2022 is under the regime with three anti-tuberculosis (Figure 2).
Figure 2: Correlation between the doses of cortisone used by the patient and the value of PLT.
Discussion
TB is ranked as the 13th leading cause of death in the world and the second leading killer of infectious origin after the COVID-19 period (on HIV / AIDS). In 2020 about 10 million people were infected with tuberculosis according to WHO reports of which 5.6 million men, 3.3 million women and 1.1 million children. TB is present in all countries and age groups.
The disease was under control especially in developing countries, until the emergence of HIV infection and the identification of resistant strains of mycobacteria [3-5]. Various hematological manifestations include abnormalities such as anemia, leukocytosis, monocytosis, lymphopenia, leukopenia, thrombocytopenia, thrombocytosis, leukomoid reactions or even pancytopenia [1,2], but the thrombocytopenia is deep in tuberculopathy and tuberculopathy there are some reports (in total 18 cases reported) [6-10].
Idiopathic thrombocytopenic purpura (ITP, also known as primary immune thrombocytopenic purpura) is an acquired disease which is defined as isolated thrombocytopenia without other potential causes present. It usually has a secret onset with a long history of purpura (purpura). > 6 months), spontaneous remission is uncommon and in most cases is likely to be incomplete [11-13]. Steroids are conventional first-line therapy for adult ITP. Platelet count increases in responding patients and usually reaches maximum values from two to four weeks after the start of treatment. However, in most patients, the platelet count drops when the steroid dose is reduced or discontinued. Another alternative treatment for primary autoimmune TPI is the use of venous immunoglobulins, the use of which is indicated in cases where certain clinical situations require an increase in immediate due to interventions or clinically significant complications [11,13]. In our case, the exclusion of ITP was done not only based on standard criteria [12], but also with the fact of the reaction of platelet value after the combination of antituberculous therapy accompanied by antituberculous therapy. Other causes of thrombocytopenia such as hemophagocytic syndrome, TTP, combined autoimmune clinical and laboratory cytopenia, bone marrow aspiration, and biopsy described in the case report were also excluded.
It is already known that various factors cause hemorrhage related to infectious conditions, of which the most common is thrombocytopenia. The etiology of thrombocytopenia in most cases appears to be an increase in platelet destruction, either due to KID or septicemia, as well as platelet adhesion to damaged vascular surfaces, or direct platelet toxicity caused by other microorganisms from the involvement of the bone marrow in the infectious process. Although the most important therapy for Infection-Related Thrombocytopenia is that directed at the underlying infection, treatment decisions for thrombocytopenic purpura immunity remain controversial and may include single or combination therapy with corticosteroids, intravenous immunoglobulin (IV). Thrombocytopenia or hemorrhage [11,14]. In the reported case the clinic was dominated by gingivorrhage, hemoptysis, diffuse petechiae and ecchymoses, macroscopic hematuria. Based on clinical examinations, radiological findings (chest x-ray and CT), demonstration of acid-fast bacilli positivity in sputum (Gene expert) and after excluding other causes of thrombocytopenia, the diagnosis of purulent immune thrombocytopenia due to TBC was made tuberculosis.
The patient was placed on corticosteroid therapy and from the follow-up to the dynamics no significant response to platelet count was observed, until the moments after the start of antituberculous therapy when the improvement of values was proven not only in laboratory but also with improved clinical performance [11,13].
The effect of steroids on thrombocytopenia is complex and has a late effect. The mechanism of action of intravenous immunoglobulins is unclear, but studies suggest blockade of Fc receptors in the cellular reticulo endothelial system and inhibition of antibody production and binding, thought to be associated with anti-idiotype antibodies that bind to antithrombotic antibodies [14]. In our case, the corticosteroids were discontinued on the 14th day of therapy and the patient was discharged from the hospital and thrombocytopenia was not detected after discontinuation of corticosteroid therapy.
These observations suggest that tuberculosis is the cause of thrombocytopenia in our patient. He had no hemolysis present, hemophagocytic syndromes, gastrointestinal hemorrhage or in other regions. Thirteen days after discharge from the hospital, the patient was clinically healthy with a platelet count of 300 × 109 / l. [15,16]. The patient is still in our follow-up without clinical or laboratory findings confirming the recurrence of thrombocytopenia. In cases where TB cases reported by the WHO are present in every corner of the world and since TB is defined as the second most common infectious cause of death in the world, the thrombocytopenic purpura in TB should remain the focus of clinicians. To clarify the exact immune mechanisms more detailed studies are needed, and they will propably enable even innovative therapies related to immune thrombocytopenia in TBC making it possible to eliminate possible side effects of corticosteroids.
References
1. https://www.who.int/teams/global-tuberculosis-programme/tb-reports
2. Kashyap R, Chaudhary VP (2003). Haematological manifestations of tuberculosis. In: Sharma SK, Mohan A, editors. Indian J Chest Dis Allied Sci. 45:247-56.
3. Ali R, Ozkalemkas F, Ozçelik T, Ozkocaman V, Ozan U, et al. (2003) Idiopathic thrombocytopenic purpura in pregnancy: A single institutional experience ëith maternal and neonatal outcomes. Ann Hematol. 82(6): 348-52.
4. Liebman HA, Pullarkat V. (2011) Diagnosis and management of immune thrombocytopenia in the era of thrombopoietin mimetics. Hematology Am Soc Hematol Educ Program . 2011:384-90.
5. Boots RJ, Roberts AE, McEvoy D. (1992) Immune thrombocytopenia complicating pulmonary tuberculosis: Case report and investigation of mechanisms. Thorax. 47(5):396-7.
6. Ursavas A, Edigar D, Ali R, Koprucuoglu D, Bahcetepe D, et al. (2010) Immune thrombocytopenia associated eith pulmonary tuberculosis. J Infect Chemother. 16(1):42-4.
7. Ghobrial ME, Albornoz MA. (2001) Immune thrombocytopenia: A rare presenting manifestation of tuberculosis. Am J Hematol. 67(2):139-43.
8. Kalra A, Kalra A, Palanisëamy C, Vikram N, Khilnani GC, et al. (2010) Immune thrombocytopenia in a challenging case of disseminated tuberculosis: A case report and revieë of the literature. Case Rep Med. 2010:946278.
9. Dagaonkar RS, Udeadia ZF. (2012) Disseminated tuberculosis eith immune thrombocytopenic purpura. Lung India. 29(1):63-5.
10. Jurak SS, Aster R, Saëaf H. (1983) Immune thrombocytopenia associated eith tuberculosis. Clin Pediatr. 22(4):318-9.
11. Cines DB, Blanchette VS. (2002) Immune thrombocytopenic purpura. N Engl J Med. 346(13):995-08.

