Epigenetic Orthodontics–Optimising Genetic Potential
Andrew Bachour*
Student, Jaume I University, Brisbane, Australia
*Corresponding author: Bachour: Andrew Bachour, Student, Jaume I University, Brisbane, Australia.
Citation: Bachour A. (2023) Epigenetic Orthodontics-Optimising Genetic Potential. J Oral Med and Dent Res. 4(1):1-17.
Received: May 10, 2023 | Published: May 31, 2023
Copyright© 2023 2023 Genesis Pub by Andrew B. This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC BY 4.0). This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author(s) and source are properly credited.
DOI: http://doi.org/10.52793/JOMDR.2023.4(1)-33
Abstract
The field of epigenetics has really come to fruition this century thanks to advances particularly in science and technology. Genetics had taken off in 1865 with Gregor Mendel’s genetic laws. The rise of epigenetics was really a consequence of the Human Genome Project carried out in the late twentieth century, a project dedicated to map the entire human genome. Initial completion took place in 2003 where approximately ninety percent of the genome had been mapped, and 2022 saw the ultimate completion of this project.
Introduction
A total of 22300 genes had been mapped out, including a large quantity of near identical repetitive segments. This was completely unexpected, initial estimates were estimating at a very minimum four times the number of genes, and likely more to explain the variation we see in the population. Additionally, of these 22300 mapped genes between humans, shows 99.9% of the sequence are identical. The variations we see amongst our species far exceeds what is possible by differences in these genes alone, and thus, the age of epigenetics was truly born. Figure 1 is a great graphic to help visualise the rapid change in epigenetics research from initial completion of the Human Genome Project [1-5].
Figure 1: Frequency of articles published against time with “epigenetics” in the title [5].
The term epigenetics was coined in the mid twentieth century by embryologist Conrad Waddington and broken down to mean ‘epi’ (above) and ‘genetics’ (gene), implying control from above the gene, as illustrated in Figure 2. We understand the genome to be our genetic blueprint, the sequence of our genes and what they do. Our epigenome on the other hand refers to how those genes are regulated. In this rapidly evolving field, many ideas or definitions are constantly superseded as our understanding grows. Currently, a widely accepted view is that epigenetics refers to mechanisms altering the phenotype or gene expression of an organism (structural and/or biochemical) without any modification of the genetic blueprint itself. This change is inheritable and may be either physiologic or pathologic [1,6].
Through years of research, we now understand the stable nature of the genome, its sequence, its heritability, and in particular, its resistance to both change and reversibility. This is in contrast to the epigenome, which is found to be highly dynamic, controlling the expression of the genome, its heritability, highly reversible nature and ability to change in response to the surrounding environment. A summary of this can be visualised in Figure 3 [8,9].
Figure 3: Genetics vs Epigenetics [8,9].
We see this everywhere in the world around us. Figure 4 is one example of this concept, where each of the four Asian rhinoceros beetles of the same species shown, appear different (body size and horn length). These changes are found due to the amount and quality of food available during their early developmental stages of life (5).
Figure 4: The Asian Rhinoceros Beetle, Trypoxylus dichotomus [5].
We see obvious changes of epigenetics in actions in ourselves. Spending time in the sun will causes melanocytes to increase their production of melanin, aiding in our protection from solar radiation. Consumption of alcohol will stimulate your body to produce more enzymes such as alcohol dehydrogenase to help clearance, Figure 5. We understand neuroplasticity, the re-organisation of our neuronal network due to environmental influences. All cells in our body have the same genetic code, yet are expressed differently, we have different cells in our body such as neurons, glial cells, osteoblasts, erythrocytes, melanocytes, adipocytes to name just a few. Without this ability to for these cells to differentiate from one another with respect to both their function and their identity, we as a species would not exist. This is the essential nature of the epigenome (10).
Figure 5: The liver responding to consumption of alcohol through epigenetic signaling [7].
Epigenetic Coding Mechanisms
When thinking of the genome and the epigenome, we need to think as if they are two distinct sets of codes. In a way the genome may be thought of as computer hardware (stable in configuration) while epigenome as the software which is running everything and constantly changing through updates. We understand the genome to be the sequence nature of deoxyribonucleic acid (DNA), nucleotide bases (adenine, cytosine, guanine, thymine) paired together to ultimately form a double helix structure. So what does the epigenome look like?
The epigenome can appear either as
- Chromatin marking
- Three dimensional structures or templates
- Self-sustaining metabolic loops
- Non-coding ribonucleic acid (RNA)
Chromatin marking and such as DNA methylation or histone modification and RNA interference are some of the most well studied of the epigenetic mechanisms. Chromatin marking may involve methylation (addition of CH3 methyl group) added to cytosine bases through the enzyme DNA methyltransferase and generally acts to silence the gene, that is to shut down transcription of the gene. The essential nature of this was demonstrated in 1992 by Rudolf Jaenisch et al, pictured in Figure 6. The research team was able to prove the importance of this through inducing a mutation of the gene encoding for DNA methyltransferase. This mutation was shown to be lethal to the embryo (11).
Figure 6: Rudolf Jaenisch. Image courtesy of Ennis et al (7).
Histone modification on the other hand is targeted to the histone, that is the protein which in which the DNA wraps around. The histone may be tagged with either a methyl group, phosphatase (PO43-) or an acetyl group (CH3CO) and may act to either activate or silence the gene. These mechanisms don’t alter the gene, rather merely tag it as shown in Figure 7 (5, 6, 9, 12).
Figure 7: Tagging the gene, not altering the sequence. Image courtesy of Ennis et al (7).
Non-coding RNA, is RNA which is not translated and is present in different forms such as small interfering, micro and piwi-interacting RNA short chains, and long non-coding RNA chains on the other hand. Although they do not code proteins, they are instrumental in gene regulation. MicroRNA short chains for example exert their influence after transcription and bind to the untranslated region of its selected messenger RNA (mRNA). Here it acts to either degrade the mRNA or inhibit its translation and been shown to be an important factor in the differentiation of osteoblasts [5,6,9,12-16].
The Link between Epigenetics and Craniofacial Development
The next natural question to ask is how does this link to craniofacial development. Naturally answers to our questions become clearer over time. Traditionally it was theorised that our genes are the sole determinant of our craniofacial development [17]. As our understanding has grown, we can now see the important over-arching epigenetic influences. Twin studies have been an important tool to recognize environmental influence. If there is a strong correlation for an identical trait to be featured by monozygotic twins for example, then genetics becomes a key factor in explaining the similarities. On the other hand, differences in monozygotic twins are better explained by epigenetic influences. It has been well established that monozygotic twins are not truly identical from a phenotypical viewpoint and quite common to see differences. Many twin studies have shown how the environment, and therefore their epigenome can be responsible for phenotypic differences rather than the genes alone [18-22]. A case study by Ramirez-Yañez showed identical twins of different appearance and compensatory oral functions look much more alike once these functions were addressed and optimised, highlighting this control beyond the gene itself [23].
Moving forward to a more functional theories, Moss introduced the functional matrix theory in the 1960s which proposed that growth of the craniofacial complex is not due to the predetermined growth of the skeletal unit itself, rather the skeletal unit is grown from the soft tissues, that is the functional matrix. It is adaptive, in response to functional demands, rather than being pre-programmed by the individual’s genetics. In this view, it is the function which can be seen to be the overriding factor in craniofacial development, ultimately controlling expression of the underlying genome [24-28]. The following decade saw Petrovic introduce the servosystem theory, based on cybernetics, and has its foundations in feedback mechanisms. It also theorized growth to be adaptive, responding to systemic influences, as well as local biomechanical/functional guidance [29,30]. Until today, we are seeing an ever more conclusive research and publications on the effects of the function and environment on form, growth and development [31-36].
Julius Wolff (1836-1902) was an early pioneer in orthopaedics and famous for his law of bone remodelling which implies that it is the function which determines the shape of the bones and went on to say that orthopaedics must be functional [37]. Some may have heard the saying, form follows function, function follows form as illustrated in Figure 8.
It is important though to add that the common mistake is to think this is a one-way street. There are cases in which genetics seems to be the overriding factor, in particular true mandibular prognathism or division 2 incisors as examples [38,39]. With the above in mind, it can be seen that neither genes or function alone is able to explain the variations noted with respect to development of the craniofacial system. It is a fine interplay between both the genome and the epigenome.
Figure 8: Julius Wolff’s law of bone remodelling.
What may be Contributing to our Epigenome Changing?
As mentioned, it is our environment which influences our epigenome. Now as we can see from Figure 9, this environment is the combination of both internal and external factors to us. There is much cross over though as the external factors will ultimately influence our internal environment. The external environment refers to the world around us, the temperature, humidity, pollution, pollens, ultraviolet radiation are examples. Our internal environment refers to within our body, the local environment within our cells and the intercellular space and how our body responds to the external environment. This may include nutrition, intracellular and intercellular chemistry, posture, habits, muscle activity, cellular signalling, and sum of the forces experienced by our cells. It can be seen that our total environment influences how our cells, and ultimately our body work.
Figure 9: Our environment is both internal and external.
The External Environment
So the question, is our external environment different today than it was 100 years ago, 10000 years ago, 100000 years ago? Little will have trouble accepting that our environment has changed markedly in this time, including my daughter who always asks me about the ‘olden days’. Three hundred thousand years ago saw the introduction of fire and therefore the transition from raw to cooked meals, twelve thousand years ago saw our species settle and develop agriculture, as opposed to moving through the land and 200 years ago saw the industrial revolution, the increased access to processed, refined foods such as sugar and flour [40,41]. These particular times in history have been shown to be associated to a change to a more disease-associated environment within the oral cavity and an increase in dental caries and periodontal disease [42-44].
So the question, is our external environment different today than it was 100 years ago, 10000 years ago, 100000 years ago? Little will have trouble accepting that our environment has changed markedly in this time, including my daughter who always asks me about the ‘olden days’. Three hundred thousand years ago saw the introduction of fire and therefore the transition from raw to cooked meals, twelve thousand years ago saw our species settle and develop agriculture, as opposed to moving through the land and 200 years ago saw the industrial revolution, the increased access to processed, refined foods such as sugar and flour [40,41]. These particular times in history have been shown to be associated to a change to a more disease-associated environment within the oral cavity and an increase in dental caries and periodontal disease [42-44].
Figure 10: Earliest scans from our species, Homo sapiens, highlights our genetic potential. Image courtesy of Max-Planck-Gesellschaft [52].
The rate of synthetic chemicals produced (chemicals which have never existed in human history) has been growing exponentially over the past one hundred years and has been shown to affect health in many ways including by affecting hormone activity [53-55]. It also doesn’t take much to see the increase in air pollution causing a reduction in the air quality we breathe in (56-58). Our modern sedentary lifestyle has also modified our inflammatory profile as highlighted in Figure 11 [59]. Table 1 and Table 2 highlight some of these changes encountered over the course of human history. In fact, many of our chronic diseases or chronic conditions are in large part due to our environment and not our genetic blueprint, and pose the question if malocclusion should be any different [60-62].
Figure 11: Sedentary lifestyle associated with a pro-inflammatory status [7].
Table 1: Changes in our nutrition over human history.
Table 2: Changes in our habits over human history.
As Figure 3 highlighted, DNA is remarkably stable and resistant to change. Without going into detail on studies of genetic evolution, the average rates of change of DNA sequence is typically measured in the base of millions of years. Changes to our genome will happen naturally over time, however, far from expeditious enough to track alongside the ever-increasing rate of change of our environment [63-66]. It is of interest to note that the first evidence of a mandibular/maxillary complex came approximately 440 million years ago and the temporomandibular joint 225 million years ago [43,67,68]. Papazian shows that assuming one in every hundred thousand wild type alleles mutates in one direction to the mutant allele every generation, then it will take nearly twelve thousand generations to increase the mutant allele from a frequency of ten to twenty percent. For the mutant gene to be seen entirely within a population, can be seen to align with the timeframes seen above [66]. Given the stable nature of the genome, our epigenome is highly dynamic and ever changing, it can therefore be seen how it must be the epigenome which is constantly responding to our changing environment, controlling expression, or lack of expression of the underlying genome.
The Internal Environment
An incredible amount of research has taken place this century looking at environmental impacts on our epigenome. In a similar fashion to the archaeological evidence discussed in the previous section, much of the pivotal research had its beginnings last century. Weston Price (1870-1948), a dentist, highlighted these environmental factors on craniofacial development. From African tribes, to Eskimos, North American Indians, Polynesians, Australian Aboriginals to Peruvian Indians, he visited the most remote of regions, where modernisation was yet to infiltrate and inhabitants living off the land. The primitive or isolated people of these lands (wear little to no clothing, great physique and live off the land) were generally free of disease, exhibited open nostrils, showed minimal tooth decay, lacked malocclusion, showed excellent bone structure with broad dental arches and all teeth present and in good alignment including third molars, also known as wisdom teeth as seen in Figure 12 (a). Areas which had been recently modernised (including introduction of imported foods such as canned food, white sugar and flour) saw a rapid deterioration in these conditions, teeth loss due to decay, severe dental and skeletal malocclusion, pinched nostrils and narrowing of the face and dental arches as seen in Figure 12 (b). The alarm was that much of this took place in the time span of a single generation, not at all predicted by genetics [69].
Figure 12: (a) Member of the west Nile tribe in Belgian Congo adopt a primitive lifestyle; (b) Native hotel staff in Belgian Congo adopting a modernised lifestyle [69].
Francis Pottenger (1901-1967), a physician, conducted a study which looked at the effects of cooked food in comparison to raw food on the dentofacial development of cats. When the cats were fed raw meat and raw milk, they maintained broad faces, well developed zygomatic and orbital arches, well developed nasal cavities, broad dental arches, and a healthy dentition. When the cats were introduced to cooked meat and pasteurised milk, their condition worsened from generation to generation, highlighting the heritability of the epigenome. These effects included narrowed zygomatic and orbital arches, mid face deficiency, longer and narrower faces, retruded mandible, impacted teeth, and malocclusion. When these cats were allowed to forage an improvement in their dental alignment was noted which was likely due to the improved nutrition and working of the muscles of mastication and facial muscles. In addition, when reintroduced to the raw meat and raw milk, improvements were seen generation after generation, until the fourth generation exhibited a normal skull and normal dental development. These show the reversible and highly dynamic nature of the epigenome [70].
The interesting conclusion with respect to these studies is made even more interesting when we realise the way at which the conclusion was reached. Price, a dentist, and Pottenger, a physician approached this in entirely separate ways only to reach the same conclusion. Where Price’s conclusions were formulated through careful observation of these populations and their behaviour, Pottenger was able to show these results experimentally. Pottenger started with healthy cats, and only through the manipulation of their diet and/or environment, was able to replicate in cats what Price had seen in these populations.
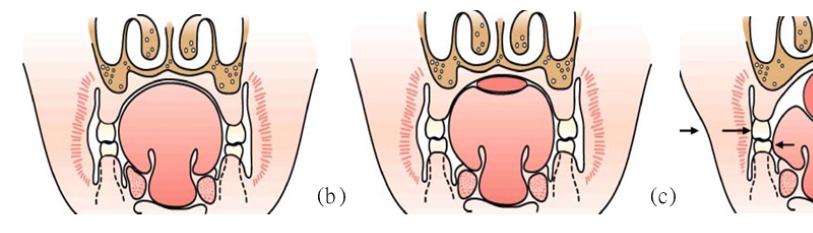
The orthodontist, Egil Harvold’s (1912-1992) primate experiments in the 1980s are another exemplification of this notion. In this experiment, primates were converted from nasal respiration to oral respiration through artificial obstruction of the nose as seen in Figure 13. This deviation in respiratory function presented obvious phenotypic differences. To begin with, an open mouth posture was seen along with altered muscle activity in comparison to the nasal respirators. This alteration in function ultimately resulted in varying types of malocclusion, narrowed mandibular arches, reduced maxillary arch length and anterior cross bite, an increase in anterior face height, increased mandibular plane angle (MPA), amongst other features [71]. This has been studied heavily with further confirmation with an open mouth posture leading to what is referred to as an adenoid face, associated with the factors of an increased MPA, retruded mandible, narrowed maxilla, increased face height and incompetent lips [72-74]. Figure 14 represents one of the pathways hypothesised in the way airway obstruction (altered function) may lead to an altered form [75].
Figure 13: (a) Optimal nasal respiration; (b) Compensatory oral respiration [71].
Muscles and connective tissues are well known to be highly plastic in nature and based on their form can result in substantially altered biomechanics [76]. Neurophysiologically, skeletal muscles are dynamic and able to change their metabolism based on environmental demands, being amount of activity or inactivity, hormonal status, loading or lack of loading. With the right training, the muscle mass or even the metabolism and ratio of fast twitch fibres (powerful but easily fatigued) to slow twitch fibres (less powerful but fatigue resistant) may be altered [77,78]. Changes in musculature have been linked to craniofacial development, it has even been said that assessing intraoral pressure may be an important tool for studying the development of malocclusions [79]. There are many examples in literature to support this association between musculature and dental or skeletal malocclusions, such as weak musculature related to increased vertical growth of the craniofacial complex or mandibular morphology linked to the muscles of mastication [80-86]. It is generally accepted that the duration of force, rather than the magnitude, is most influential with respect to structural form, that is, longer acting rest postures, although less powerful, can produce greatest structural changes, as opposed to forces which are higher impact but short lived [87, 88].
Figure 14: One of the proposed mechanisms behind nasopharyngeal obstruction [75].
Studies have been performed in infants looking at the difference in muscle activity between breastfeeding and artificial forms of feeding. It has been shown the masseter and temporalis muscles are less active during bottle feeding in comparison to breast feeding. With the safe assumption that perfect breastfeeding biomechanics recruits the perfect muscles necessary, then it has been shown that bottle feeding leads to a reduction of activity in the tongue, lips, temporalis muscle, masseter muscle and the pterygoids, with increased activity of both the mentalis and buccinator [89-91]. The differences in tongue position can be clearly seen between breastfeeding and bottle feeding in Figure 15, and this consequently must result in altered function.
Ankyloglossia, also commonly known as tongue tie, is a condition which may affect function of the tongue causing restriction in movement and consequently results in poor biomechanics. Additionally, restriction of the upper lip will additionally cause altered function and poor biomechanics, including open lip posture. Releasing the tethered tissue in these cases of tethered tissue causing a functional deficit, has shown to be advantageous with respect to function of the muscle [92-95]. Ankyloglossia has also been shown to be correlated with underdevelopment of the maxilla, amongst other dental and skeletal changes [96,97]. As seen previously, any deviation from optimal function may ultimately result in consequences to the craniofacial system [98-100].
Figure 15: Tongue position (a) at rest (b) during breastfeeding (c) during bottle feeding [101].
Optimising Genetic Potential
One may now ask, what does it mean to optimise genetic potential. According to the Cambridge Dictionary, potential is defined as either possible when the conditions necessary are met, or as the ability to develop [102]. As we saw earlier, it is not that we have lost the genes necessary to develop a well-formed craniofacial structure. In fact, we still have the genes necessary, however, we have just altered their expression. We can also see that optimised health is associated with optimised development.
In lieu of the above, it can be seen that ideal function is necessary to ensure ideal genetic potential is reached. Any compensatory action will cause a deviation from the optimal potential through the epigenome. From infancy, we need to consider many environmental and functional aspects when discussing optimal development with respect to the epigenome, including:
- Maternal and paternal health preconception [103-106]
- Maternal health during pregnancy [107,108]
- Delivery method [109,110]
- Breastfeeding [111,112)
- Nutrition (whole foods, organic, harder diet)
- Functions (soft tissue restriction (incl. fascia), swallowing, chewing, baby led weaning)
- Posture (resting tongue posture, resting lip posture, muscle balance)
- Movement (physical activity, full body biomechanics)
- Breathing (neuromuscular, biomechanical, biochemical, psychophysiological) [113,114]
- Sleep Quality (sleep hygiene, sleep position, sleep architecture) [115,116]
Conclusion
It can be seen that the relationship between the genome and the epigenome is not in fact dichotomic, but rather complimentary. Ultimately, we can see the close link to our environment around us and within us. The more we can do to function in an ideal way, the more our form will mirror this through the control of gene expression. Ideally, we want the sum of our environment, functions and underlying genetics to produce a great symphony orchestra. If Wolff’s law is taken one step further, it may be stated that ideal form will support ideal function, whereas compensatory form will support compensatory function. With respect to craniofacial development, we need to take into account not only the underlying genetics of the individual, but rather the entire epigenome. In such way, treatment must be focused in a way to address the underlying cause of the malocclusion, and in doing so, ensure best long term health outcomes for the patient.
References
- Tollefsbol T. (2017) Handbook of Epigenetics: The New Molecular and Medical Genetics: Elsevier Science.
- https://www.genome.gov/human-genome-project.
- https://www.genome.gov/about-genomics/educationalresources/fact-sheets/human-genome-project.
- https://www.genome.gov/sites/default/files/media/files/202009/HGP_Timeline.pdf.
- Baedke J. (2018) Above the Gene, Beyond Biology: Toward a Philosophy of Epigenetics: University of Pittsburgh Press.
- Li Y. (2021) Modern epigenetics methods in biological research. Methods. 187:104-13.
- Ennis C, Pugh O. (2017) Introducing Epigenetics: A Graphic Guide: Icon Books Limited.
- Janmeda M, Bayan J, editors. (2017) Epigenetics: Regulation of Gene Expression.
- James S, Shubhalakshmi S, Koraprath S. (2020) Epigenetics in Oral Pathology. IJAR. 8:817-30.
- Ballestar E. (2011) An introduction to epigenetics. Epigenetic Contributions in Autoimmune Disease. 1-11.
- Li E, Bestor TH, Jaenisch R. (1992) Targeted mutation of the DNA methyltransferase gene results in embryonic lethality. Cell. 69(6):915-26.
- Williams SD, Hughes TE, Adler CJ, Brook AH, Townsend GC. (2014) Epigenetics: a new frontier in dentistry. Aust Dent J. 59 (Suppl 1):23-33.
- Holoch D, Moazed D. (2015) RNA-mediated epigenetic regulation of gene expression. Nat Rev Genet. 16(2):71-84.
- Seo JY, Park YJ, Yi YA, Hwang JY, Lee IB, et al. (2015) Epigenetics: general characteristics and implications for oral health. Restor Dent Endod. 40(1):14-22.
- Perez P, Jang SI, Alevizos I. (2014) Emerging landscape of non-coding RNAs in oral health and disease. Oral Dis. 20(3):226-35.
- Sun Q, Liu H, Chen Z. (2015) The fine-tuning role of microRNA-RNA interaction in odontoblast differentiation and disease. Oral Dis. 21(2):142-8.
- Brodie AG. (1941) On the growth pattern of the human head. From the third month to the eighth year of life. American Journal of Anatomy. 68(2):209-62.
- Varela M, Trujillo-Tiebas M, Garcia-Camba P. (2011) Identical twins revealing discordant hypodontia. The rationale of dental arch differences in monozygotic twins. EJPD. 12(6):318-22.
- Townsend GC, Pinkerton SK, Rogers JR, Bockmann MR, Hughes TE. (2015) Twin studies: research in genes, teeth and faces: University of Adelaide Press.
- Kawala B, Antoszewska J, Necka A. (2007) Genetics or environment? A twin-method study of malocclusions. World J Orthod. 8(4):405-10.
- Lobb WK. (1987) Craniofacial morphology and occlusal variation in monozygous and dizygous twins. Angle Orthod. 57(3):219-33.
- Patel V, Preedy V. (2019) Handbook of Nutrition, Diet, and Epigenetics.
- Ramirez-Yañez G. (2019) Craniofacial Growth and Development. In: Liem E, editor. Sleep Disorders in Pediatric Dentistry: Springer.
- Moss ML, Salentijn L. (1969) The primary role of functional matrices in facial growth. Am J Orthod. 55(6):566-77.
- Moss ML. (1997) The functional matrix hypothesis revisited. 1. The role of mechanotransduction. Am J Orthodo Dentofacial Orthop. 112(1):8-11.
- Moss ML. (1997) The functional matrix hypothesis revisited. 2. The role of an osseous connected cellular network. Am J Orthodo Dentofacial Orthop. 112(2):221-6.
- Moss ML. (1997) The functional matrix hypothesis revisited. 3. The genomic thesis. Am J Orthodo Dentofacial Orthop. 112(3):338-42.
- Moss ML. (1997) The functional matrix hypothesis revisited. 4. The epigenetic antithesis and the resolving synthesis. Am J Orthodo Dentofacial Orthop. 112(4):410-7.
- Carlson D. (2005) Theories of Craniofacial Growth in the Postgenomic Era. Seminars in Orthodontics. 11:172-83.
- Petrovic A. (1984) Experimental and Cybernetic Approaches to the Mechanism of Action of Functional Appliances on Mandibular Growth. In: McNamara JA, Ribbens KA, editors. Malocclusion and the Periodontium: Center for Human Growth and Development, The University of Michigan.
- Kiliaridis S, Engström C, Chavez LME. (1992) Influence of masticatory muscle function on craniofacial growth in hypocalcemic rats. EJOS. 100(6):330-36.
- Rahme J, el-Danaf A, Chassagne JF, Maxant P, Stricker M, et al. (1987) The influence of the masticatory muscles on craniofacial growth. A microsurgical study in the rat. Rev Stomatol Chir Maxillofac. 88(2):108-15.
- Marghoub A, Libby J, Babbs C, Pauws E, Fagan MJ. (2018) Predicting calvarial growth in normal and craniosynostotic mice using a computational approach. J Anat. 232(3):440-8.
- Henderson JH, Longaker MT, Carter DR. (2004) Sutural bone deposition rate and strain magnitude during cranial development. Bone. 34(2):271-80.
- Xavier AM, Chandra HS, Vijay M. (2022) Craniofacial Growth and Development in Children. Illustrated Pediatric Dentistry (Part I).
- Rengasamy Venugopalan S, Allareddy V. (2022) Craniofacial Growth and Development. Peterson’s Principles of Oral and Maxillofacial Surgery: Springer.1729-65.
- Wolff J. (1986) The Law of Bone Remodelling. Springer, Berlin.
- Xue F, Wong RW, Rabie AB. (2010) Genes, genetics, and Class III malocclusion. Orthod Craniofac Res. 13(2):69-74.
- Markovic MD. (1992) At the crossroads of oral facial genetics. Eur J Orthod. 14(6):469-81.
- Harari YN. (2015) Sapiens: a brief history of humankind.
- Cordain L, Eaton SB, Sebastian A, Mann N, Lindeberg S, et al. (2005) Origins and evolution of the Western diet: health implications for the 21st century. Am J Clin Nutr. 81(2):341-54.
- Adler CJ, Dobney K, Weyrich LS, Kaidonis J, Walker AW, et al. (2013) Sequencing ancient calcified dental plaque shows changes in oral microbiota with dietary shifts of the Neolithic and Industrial revolutions. Nature Genetics. 45(4):450-5.
- Nowzari H, Jorgensen M. (2022) Human Dento-Facial Evolution: Cranial Capacity, Facial Expression, Language, Oral Complications and Diseases. Oral. 2(2):163-72.
- Latham K. (2013) Human Health and the Neolithic Revolution: An Overview of Impacts of the Agricultural Transition on Oral Health, Epidemiology, and the Human Body. Nebraska Anthropologist.
- Sardi M, Ramirez Rozzi F, Pucciarelli H. (2004) The Neolithic transition in Europe and North Africa. The functional craneology contribution. Anthropol Anz. 62(2):129-45.
- Weltman A, Weltman JY, Veldhuis JD, Hartman ML. (2001) Body composition, physical exercise, growth hormone and obesity. Eat Weight Disord. 6(3):28-37.
- Barr SI, McKay HA. (1998) Nutrition, exercise, and bone status in youth. Int J Sport Nutr. 8(2):124-42.
- Banu J, Orhii P, Okafor M, Wang L, Kalu D. (2001) Analysis of the effects of growth hormone, exercise and food restriction on cancellous bone in different bone sites in middle-aged female rats. Mech ageing dev. 122(8):849-64.
- Larsen CS. (2006) The agricultural revolution as environmental catastrophe: Implications for health and lifestyle in the Holocene. J quaint. 150(1):12-20.
- Larsen CS. (1981) Skeletal and Dental Adaptations to the Shift to Agriculture on the Georgia Coast. Current Anthropology. 122:422-3.
- Eshed V, Gopher A, Pinhasi R, Hershkovitz I. (2010) Paleopathology and the origin of agriculture in the Levant. Am J Phys Anthropol. 143(1):121-33.
- https://www.mpg.de/11322481/oldest-homo-sapiens-fossils-at-jebel-irhoud-morocco
- Naidu R, Biswas B, Willett IR, Cribb J, Kumar SB, et al. (2021) Chemical pollution: A growing peril and potential catastrophic risk to humanity. Environment International. 156:106616.
- Binetti R, Costamagna FM, Marcello I. (2008) Exponential growth of new chemicals and evolution of information relevant to risk control. Ann Ist Super Sanita. 44(1):13-5.
- Schug TT, Janesick A, Blumberg B, Heindel JJ. (2011) Endocrine disrupting chemicals and disease susceptibility. J Steroid Biochem Mol Biol. 127(35):204-15.
- Avol EL, Gauderman WJ, Tan SM, London SJ, Peters JM. (2001) Respiratory Effects of Relocating to Areas of Differing Air Pollution Levels. Am J Respir Crit Care Med. 164(11):2067-72.
- Bates DV. (1995) The effects of air pollution on children. Environ Health Perspect. 103(6):49-53.
- Chen B, Kan H. (2008) Air pollution and population health: a global challenge. Environ Health Prev Med. 13(2):94-101.
- Phillips CM, Dillon CB, Perry IJ. (2017) Does replacing sedentary behaviour with light or moderate to vigorous physical activity modulate inflammatory status in adults? Int J Behav Nutr and Phys Act. 14(1):138.
- Willett W, Koplan J, Nugent R, Dusenbury C, Puska P, et al. (2006) Prevention of Chronic Disease by Means of Diet and Lifestyle Changes. Disease Control Priorities in Developing Countries. 2nd edition.
- McGuinn LA, Ghazarian AA, Ellison GL, Harvey CE, Kaefer CM, et al. (2012) Cancer and environment: definitions and misconceptions. Environ Res. 112:230-4.
- McGuinn L, Ghazarian A, Ellison G, Harvey C, Kaefer C, et al. (2011) Cancer and environment: Definitions and misconceptions. Environ Res. 112:230-4.
- Britten RJ. (1986) Rates of DNA sequence evolution differ between taxonomic groups. Science. 231(4744):1393-8.
- Gillooly JF, Allen AP, West GB, Brown JH. (2005) The rate of DNA evolution: effects of body size and temperature on the molecular clock. Proceedings of the National Academy of Sciences. 102(1):140-5.
- Lanave C, Preparata G, Sacone C, Serio G. (1984) A new method for calculating evolutionary substitution rates. J Mol Evol. 20(1):86-93.
- Papazian HP, Crew FAE. (1967) Modern genetic. London: Weidenfeld and Nicolson.
- Anthwal N, Joshi L, Tucker AS. (2013) Evolution of the mammalian middle ear and jaw: adaptations and novel structures. J Anat. 222(1):147-60.
- Rücklin M, Donoghue PCJ, Johanson Z, Trinajstic K, Marone F, et al. Development of teeth and jaws in the earliest jawed vertebrates. Nature. 491(7426):748-51.
- Price WA. (2016) Nutrition and Physical Degeneration: A Comparison of Primitive and Modern Diets and Their Effects. 8th ed.
- Pottenger FM. (2012) Pottenger's Cats: A Study in Nutrition. California: Price-Pottenger Nutrition Foundation.
- Harvold EP, Tomer BS, Vargervik K, Chierici G. (1981) Primate experiments on oral respiration. AJO-DO. 79(4):359-72.
- Peltomäki T. (2007) The effect of mode of breathing on craniofacial growth--revisited. Eur J Orthod. 29(5):426-9.
- Koca CF, Erdem T, Bayındır T. (2016) The effect of adenoid hypertrophy on maxillofacial development: an objective photographic analysis. J Otolaryngol Head Neck Surg. 45(1):48.
- Tourne LPM. (1990) The long face syndrome and impairment of the nasopharyngeal airway. Angle Orthod. 60(3):167-76.
- Solow B, Kreiborg S. (1977) Soft-tissue stretching: a possible control factor in craniofacial morphogenesis. Scand J Dent Res. 85(6):505-7.
- Standerwick RG, Roberts WE. (2009) The aponeurotic tension model of craniofacial growth in man. Open Dent J. 3:100-13.
- Pimenidis M. (2009) The Neurobiology of Orthodontics: Treatment of Malocclusion Through Neuroplasticity.
- Baldwin KM, Haddad F. (2019) The Evolution of Skeletal Muscle Plasticity in Response to Physical Activity and Inactivity. Muscle and Exercise Physiology. 347-77.
- Engelke W, Jung K, Knösel M. (2011) Intra-oral compartment pressures: a biofunctional model and experimental measurements under different conditions of posture. Clin Oral Investig. 15(2):165-76.
- Kiliaridis S, Mejersjö C, Thilander B. (1989) Muscle function and craniofacial morphology: a clinical study in patients with myotonic dystrophy. Eur J Orthod. 11(2):131-8.
- Ruan WH, Su JM, Ye XW. (2007) Pressure from the lips and the tongue in children with class III malocclusion. J Zhejiang Univ Sci B. 8(5):296-01.
- Yamada T, Sugiyama G, Mori Y. (2020) Masticatory muscle function affects the pathological conditions of dentofacial deformities. Jpn Dent Sci Rev. 56(1):56-61.
- Avis V. (1959) The relation of the temporal muscle to the form of the coronoid process. Am J Phys Anthropol. 17(2):99-104.
- Maki K, Miller AJ, Okano T, Hatcher D, Yamaguchi T, et al. (2001) Cortical bone mineral density in asymmetrical mandibles: a three-dimensional quantitative computed tomography study. Eur J Orthod. 23(3):217-32.
- Becht MP, Mah J, Martin C, Razmus T, Gunel E, et al. (2014) Evaluation of masseter muscle morphology in different types of malocclusions using cone beam computed tomography. Int Orthod. 12(1):32-48.
- Nielsen IL. (1991) Vertical malocclusions: etiology, development, diagnosis and some aspects of treatment. Angle Orthod. 61(4):247-60.
- Proffit WR. (1978) Equilibrium theory revisited: factors influencing position of the teeth. Angle Orthod. 48(3):175-86.
- Mew JR. (2004) The postural basis of malocclusion: a philosophical overview. Am J Orthod Dentofacial Orthop. 126(6):729-38.
- Gomes CF, Trezza EM, Murade EC, Padovani CR. (2006) Surface electromyography of facial muscles during natural and artificial feeding of infants. J Pediatr (Rio J). 82(2):103-9.
- Inoue N, Sakashita R, Kamegai T. (1995) Reduction of masseter muscle activity in bottle-fed babies. Early Hum Dev. 42(3):185-93.
- França ECL, Sousa CB, Aragão LC, Costa LR. (2014) Electromyographic analysis of masseter muscle in newborns during suction in breast, bottle or cup feeding. BMC Pregnancy and Childbirth. 14(1):154.
- Geddes DT, Langton DB, Gollow I, Jacobs LA, Hartmann PE, et al. (2008) Frenulotomy for breastfeeding infants with ankyloglossia: effect on milk removal and sucking mechanism as imaged by ultrasound. Pediatrics. 122(1):188-94.
- Ghaheri BA, Cole M, Fausel SC, Chuop M, Mace JC. (2017) Breastfeeding improvement following tongue-tie and lip-tie release: A prospective cohort study. The Laryngoscope. 127(5):1217-23.
- Ghaheri BA, Lincoln D, Mai TNT, Mace JC. (2022) Objective Improvement After Frenotomy for Posterior Tongue-Tie: A Prospective Randomized Trial. Otolaryngology Head and Neck Surgery. 166(5):976-84.
- Seo YJ, Kim SJ, Munkhshur J, Chung KR, Ngan P, et al. (2014) Treatment and retention of relapsed anterior open-bite with low tongue posture and tongue-tie: A 10-year follow-up. Korean J Orthod. 44(4):203-16.
- Yoon AJ, Zaghi S, Ha S, Law CS, Guilleminault C, et al. (2017) Ankyloglossia as a risk factor for maxillary hypoplasia and soft palate elongation: A functional - morphological study. Orthod Craniofac Res. 20(4):237-44.
- Srinivasan B, Chitharanjan AB. (2013) Skeletal and dental characteristics in subjects with ankyloglossia. Prog Orthod. 14:44.
- Ovsenik M. (2009) Incorrect orofacial functions until 5 years of age and their association with posterior crossbite. Am J Orthod Dentofacial Orthop. 136(3):375-81.
- D'Onofrio L. (2019) Oral dysfunction as a cause of malocclusion. Orthodontics & Craniofacial Research. 22(S1):43-8.
- Lavergne J, Petrovic A. (1983) Discontinuities in occlusal relationship and the regulation of facial growth. A cybernetic view. Eur J Orthod. 5(4):269-78.
- https://www.brianpalmerdds.com/pdf/adsm_section_c.pdf
- https://dictionary.cambridge.org/dictionary/english/potential
- Zalbahar N, Najman J, McIntrye HD, Mamun A. (2016) Parental pre-pregnancy BMI influences on offspring BMI and waist circumference at 21 years. Aust N Z J Public Health. 40(6):572-8.
- Casas M, Chatzi L, Carsin A-E, Amiano P, Guxens M, et al. (2013) Maternal prepregnancy overweight and obesity, and child neuropsychological development: two Southern European birth cohort studies. Int J Epidemiol. 42(2):506-17.
- Dunford AR, Sangster JM. (2017) Maternal and paternal periconceptional nutrition as an indicator of offspring metabolic syndrome risk in later life through epigenetic imprinting: A systematic review. Diabetes & Metabolic Syndrome: Clinical Research & Reviews. Diabetes Metab Syndr. 11:655-62.
- Marcho C, Oluwayiose OA, Pilsner JR. (2020) The preconception environment and sperm epigenetics. Andrology. 8(4):924-42.
- Knopik VS, Maccani MA, Francazio S, McGeary JE. (2012) The epigenetics of maternal cigarette smoking during pregnancy and effects on child development. Dev Psychopathol. 24(4):1377-90.
- Agarwal P, Morriseau TS, Kereliuk SM, Doucette CA, Wicklow BA, et al. (2018) Maternal obesity, diabetes during pregnancy and epigenetic mechanisms that influence the developmental origins of cardiometabolic disease in the offspring. Crit Rev Clin Lab Sci. 55(2):71-101.
- Rahman M, Khan N, Rahman A, Alam M, Khan A. (2022) Long-term effects of caesarean delivery on health and behavioural outcomes of the mother and child in Bangladesh. J Health Popul Nutr. 41(1):45.
- Chen H, Tan D. (2019) Cesarean section or natural childbirth? Cesarean birth may damage your health. Front Psychol. 10:351.
- Salone LR, Vann WF, Dee DL. (2013) Breastfeeding: An overview of oral and general health benefits. The J Am Det Assoc. 144(2):143-51.
- Turck D, Vidailhet M, Bocquet A, Bresson J, Briend A, et al. (2013) Breastfeeding: health benefits for child and mother. Archives de pediar. 20:29-48.
- Courtney R. (2009) The functions of breathing and its dysfunctions and their relationship to breathing therapy. International Journal of Osteopathic Medicine. 12(3):78-85.
- Benner A, Patel AK, Singh K, Dua A. (2008) Physiology, Bohr Effect.
- Ohayon M, Wickwire EM, Hirshkowitz M, Albert SM, Avidan A, et al. (2017) National Sleep Foundation's sleep quality recommendations: first report. Sleep Health. 3(1):6-19.
- Kryger MH, Roth T, Dement WC. (2017) Principles and Practice of Sleep Medicine.

